Question
Question: There is no ring strain in cyclohexane, but cyclobutane has an angle strain of \({{9}^{{}^\circ }}{{...
There is no ring strain in cyclohexane, but cyclobutane has an angle strain of 9∘44′. If ΔHc∘of cyclohexane per (CH2) group is 660 KJ/mol and ΔHc∘ of cyclobutane is 2744 KJ/mol, what is the ring strain in KJ/mol for cyclobutane?
[A] -104
[B] 104
[C] -2084
[D] 2084
Solution
To solve this question, we can use the ΔHc∘ value of each (CH2) groups and the cyclobutane. We can find out the ring strain by subtracting the ΔHc∘ value of each (CH2)group present in cyclobutane by the ΔHc∘ value of cyclobutane. The total number of (CH2) groups in cyclobutane is four.
Complete answer:
We know that ring strain in organic molecules is a form of instability that exists when the angles formed between the molecules are unstable.
We observe ring strain generally in smaller organic molecules like cyclobutane and cyclopropane.
In the given question, we have that the cyclobutane has an angle strain of 9∘44′. We know that there are four CH2 groups in cyclobutane and we can draw its structure as-
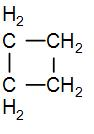
As we can see from the above diagram that there are 4 CH2 groups. So to find the ring strain in cyclobutane, we can subtract the standard enthalpy of combustion of each of these CH2 groups from the standard enthalpy of combustion of cyclobutane.
Therefore, we can write that-
Ring strain = 4×ΔHc∘ of each CH2 group.-ΔHc∘of cyclobutane
Therefore, ring strain = (4×660)KJ/mol - 2274 KJ/mol =140 KJ/mol.
From the above calculation it is clear that ring strain in a cyclobutane ring is 104 KL/mol.
So, the correct answer is “Option B”.
Note: Ring strain is mostly caused due to the sp3 hybridised carbons which are present in the cycloalkanes. For a ring to not be strained the ideal angle is 109.5∘ but due to the sp3 hybridised carbons, the bond angle is not maintained thus causes strain in rings.
