Question
Question: There is a mixture of p-hydroxy benzoic acid and p-amino benzoic acid. Discuss one method by which w...
There is a mixture of p-hydroxy benzoic acid and p-amino benzoic acid. Discuss one method by which we can separate them and also write down the confirmatory test of the functional groups present.
Solution
Binary mixture is the mixture of two compounds which are immiscible with each other. They may be homogenous or heterogenous Preliminary tests for the separation of binary mixtures are based on the state, odor and color of the mixture.
Complete step by step solution:
Initial identification of two compounds from a given binary mixture of organic compounds qualitatively and identification of its compounds can be determined on the basis on nature of binary mixtures and type of binary mixtures.
The preliminary test includes action of HCl with the mixture. The structures of p-hydroxy benzoic acid and p-amino benzoic acid are given below:
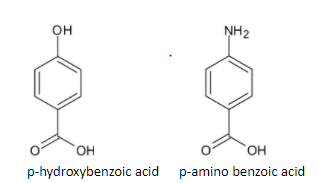
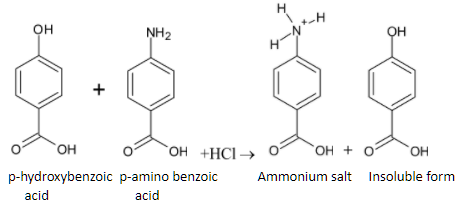
When the mixture is reacted with HCl, p-hydroxybenzoic acid is not soluble while p-amino benzoic acid dissolves in the solution. It forms ammonium salt. The reaction is given below:
When both the compounds are reacted with sodium bicarbonate, it produces brisk effervescence which is due to the carbon dioxide gas. The reaction is given below:
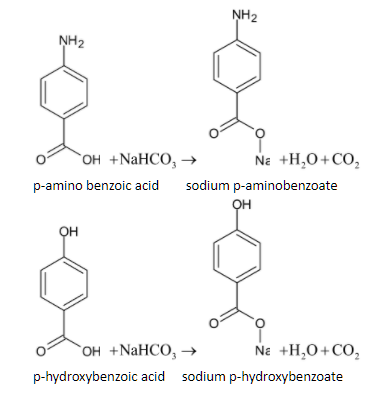
Thus it can be separated easily.
The functional groups can be confirmed by azo dye test and Libermann nitroso test. In an azo dye test, when p-amino benzoic acid is reacted with nitrous acid, HONO to give diazonium salt. In Libermann test, when p-hydroxybenzoic acid is reacted with nitrous acid, HONO to give blue or green color.
Note: Amino groups can also be determined by carbylamine test. It is also called an isocyanide test. The reaction involves action with chloroform in the presence of alkali. It produces isocyanide, hence the name isocyanide test. Aliphatic and aromatic amines give positive results for carbylamine test.
