Question
Question: There are some compounds which are as follow: \[\;1 - hexene,{\text{ }}2 - methyl - 1 - pentene,{\...
There are some compounds which are as follow:
1−hexene, 2−methyl−1−pentene, 2−hexene
Which of the following is the correct order for increasing order of reactivity for acid catalyzed hydration?
A.1−hexene< 2−hexene<2−methyl−1−pentene
B.2−hexene<1−hexene<2−methyl−1−pentene
C.2−methyl−1−pentene<1−hexene<2−hexene
Solution
A carbocation is a molecule wherein a carbon atom has an effective fee and 3 bonds. We can essentially say that they may be carbon cations. Formerly, it changed into referred to as carbon atom having a positive charge. Carbocation nowadays is described as any even-electron cation that possesses a large effective fee at the carbon atom.
Complete Answer:
A carbocation is an ion with an in reality charged carbon atom. Among the handiest examples are methenium, methanium and vinyl cations. Occasionally, carbocations that undergo a couple of in reality charged carbon atoms also are encountered. In the present-day definition given via way of the IUPAC, a carbocation is any even-electron cation with a superb partial superb charge on a carbon atom. They are in addition categorized in crucial commands steady with the coordination quantity of the charged carbon: three internal sides the carbenium ions and five inside facet the carbonium ions. The reactivity order is primarily based totally upon the stableness of carbocation. The extra strong the carbocation, extra might be the stableness. So the excellent order might be:
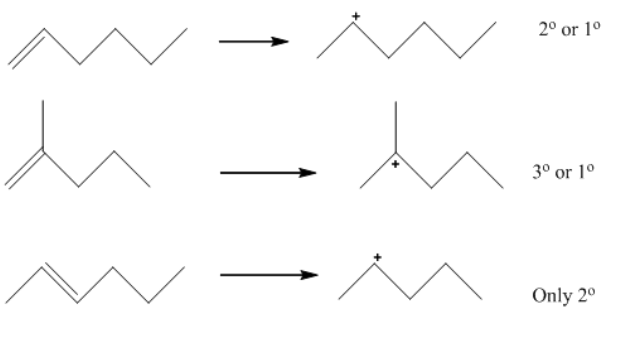
Option A is the correct answer.
Note:
Carbon having a positive charge, is on the begin defined through manner of way of Olah, are characterized thru manner of way of a three-middle -electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations. Carbocation turns into extra solid than carbanion because of the presence of three donor methyl corporations which donate electrons and consequently significantly stabilize the+charge.
