Question
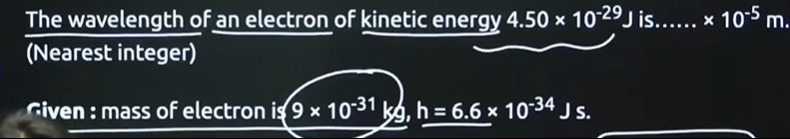
Question: The wavelength of an electron of kinetic energy 4.50 × 10-29 J is...... × 10-5 m. (Nearest integer)...
The wavelength of an electron of kinetic energy 4.50 × 10-29 J is...... × 10-5 m. (Nearest integer)
7
Solution
The de Broglie wavelength (λ) of a particle is given by the formula λ=ph, where h is Planck's constant and p is the momentum of the particle. Momentum can be related to kinetic energy (KE) using the formula p=2mKE, where m is the mass of the particle. Substituting this into the de Broglie equation gives: λ=2mKEh Given values are: Planck's constant, h=6.6×10−34 J s Mass of electron, m=9×10−31 kg Kinetic energy, KE=4.50×10−29 J
Substitute these values into the formula:
λ=2×(9×10−31 kg)×(4.50×10−29 J)6.6×10−34 J s
First, calculate the term inside the square root:
2mKE=2×9×10−31×4.50×10−29=81×10−60 kg2m2/s2
Now, take the square root:
2mKE=81×10−60=9×10−30 kg m/s
Now, calculate the wavelength:
λ=9×10−30 kg m/s6.6×10−34 J s=96.6×10−34−(−30) m
λ=96.6×10−4 m
Calculating the fraction:
96.6≈0.7333...
So, the wavelength is:
λ≈0.7333...×10−4 m
The question asks for the answer in the format ...... × 10-5 m. Convert the wavelength to this format:
λ≈0.7333...×10−4 m=7.333...×10−5 m
The question requires the nearest integer value for the blank space. The value is 7.333.... The nearest integer to 7.333... is 7.
