Question
Question: The variation of molar conductivity with concentration of an electrolyte (X) in aqueous solution is ...
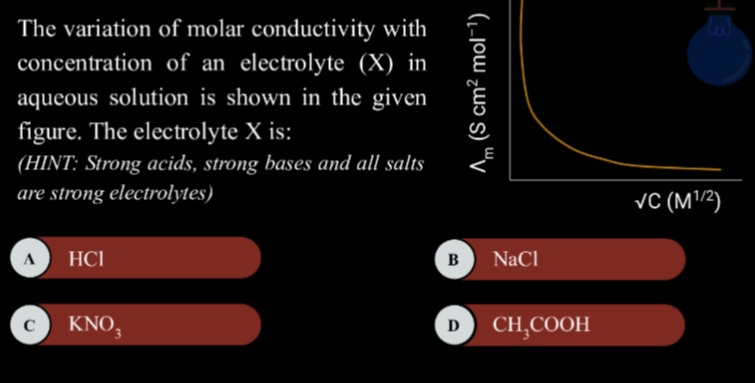
The variation of molar conductivity with concentration of an electrolyte (X) in aqueous solution is shown in the given figure. The electrolyte X is:(HINT: Strong acids, strong bases and all salts are strong electrolytes)
HCl
NaCl
KNO3
CH3COOH
CH3COOH
Solution
The variation of molar conductivity (Λm) with concentration for electrolytes is different for strong and weak electrolytes.
For strong electrolytes, the molar conductivity decreases with increasing concentration due to increased interionic attractions. At infinite dilution (zero concentration), the interionic attractions are negligible, and the molar conductivity reaches a limiting value (Λm0). The variation of molar conductivity with the square root of concentration (C) is approximately linear at low concentrations and is described by the Debye-Hückel-Onsager equation:
Λm=Λm0−AC
where A is a constant. The plot of Λm versus C for a strong electrolyte is a straight line with a negative slope.
For weak electrolytes, the degree of dissociation (α) is less than 1 and increases with dilution. The molar conductivity is given by Λm=αΛm0. As the concentration decreases (dilution increases), the degree of dissociation increases, leading to a significant increase in the number of ions in the solution and hence a significant increase in molar conductivity. At very high dilutions, the degree of dissociation approaches 1, and Λm approaches Λm0. The plot of Λm versus C for a weak electrolyte is a non-linear curve that shows a steep increase in Λm as C approaches zero.
The given graph shows a steep, non-linear increase in molar conductivity as the square root of concentration decreases, which is characteristic of a weak electrolyte.
Now let's examine the given options:
A. HCl is a strong acid, which is a strong electrolyte. B. NaCl is a salt of a strong acid and a strong base, which is a strong electrolyte. C. KNO3 is a salt of a strong acid and a strong base, which is a strong electrolyte. D. CH3COOH is acetic acid, which is a weak acid and therefore a weak electrolyte.
Since the graph shows the behavior of a weak electrolyte, the electrolyte X is CH3COOH.
