Question
Question: The vapour pressures of A and B at 25°C are 90 mm Hg and 15 mm Hg respectively. If A and B are mixed...
The vapour pressures of A and B at 25°C are 90 mm Hg and 15 mm Hg respectively. If A and B are mixed such that the mole fraction of A in the mixture is 0.6, then the mole fraction of B in the vapour phase is x×10−1. The value of x is ___. (Nearest integer)
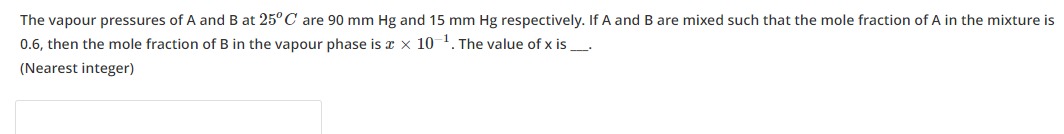
1
Solution
The vapor pressures of pure A and B are given as PA0=90 mm Hg and PB0=15 mm Hg.
The mole fraction of A in the liquid mixture is xA=0.6.
The mole fraction of B in the liquid mixture is xB=1−xA=1−0.6=0.4.
According to Raoult's Law, the partial pressure of a component in the vapor phase above the solution is given by:
PA=xA×PA0
PB=xB×PB0
Substitute the given values:
PA=0.6×90 mm Hg=54 mm Hg
PB=0.4×15 mm Hg=6 mm Hg
According to Dalton's Law of partial pressures, the total pressure of the solution is the sum of the partial pressures:
Ptotal=PA+PB
Ptotal=54 mm Hg+6 mm Hg=60 mm Hg
The mole fraction of a component in the vapor phase is given by the ratio of its partial pressure to the total pressure:
yB=PtotalPB
yB=60 mm Hg6 mm Hg=101=0.1
The problem states that the mole fraction of B in the vapor phase is x×10−1.
So, yB=x×10−1.
We have calculated yB=0.1.
0.1=x×10−1
0.1=x×0.1
x=0.10.1=1
The value of x is 1. The nearest integer value of x is 1.
