Question
Question: The vapour pressure curves of the same solute in the same solvent are shown below. The curves are pa...
The vapour pressure curves of the same solute in the same solvent are shown below. The curves are parallel to each other and do not intersect. The concentrations of solutions are in order of:
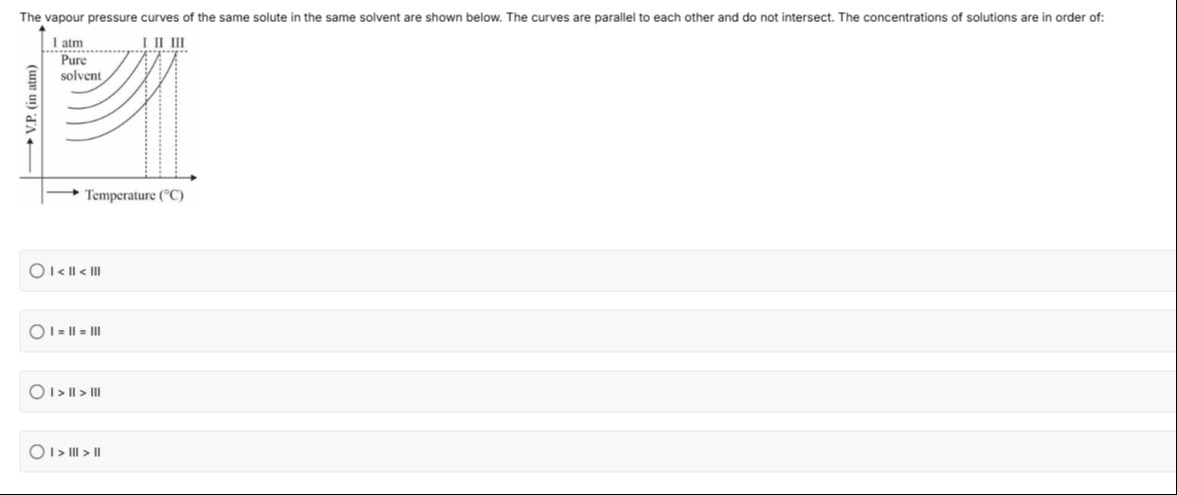
I < II < III
I = II = III
I > II > III
I > III > II
I < II < III
Solution
The vapour pressure of a solution is lower than that of the pure solvent, and this lowering is directly proportional to the solute concentration. From the graph, at any given temperature, the vapour pressure follows the order: Pure solvent > I > II > III. This means that solution III exhibits the greatest lowering of vapour pressure, followed by II, and then I. Consequently, the concentration of solution III is the highest, and the concentration of solution I is the lowest. Thus, the order of concentrations is I < II < III.
