Question
Question: The value for crystal field stabilisation energy is zero for: (A) \({ K }_{ 2 }\left[ Mn{ F }_{ 6 ...
The value for crystal field stabilisation energy is zero for:
(A) K2[MnF6]
(B) K3[Fe(CN)6]
(C) K3[FeF6]
(D) K4[Fe(CN)6]
Solution
Hint: Crystal field stabilization energy: Crystal field stabilization energy is the energy of electronic configuration in the ligand field - Electronic configuration in the isotropic field.
Complete answer step-by-step:
The splitting pattern in each complex compound is octahedral. If a strong field ligand is present, then back pairing will take place as in the case of B and D, and splitting energy can never be zero, since no electron can go into eg orbitals.
But if a strong field is not present, then back pairing will not take place as in the case of A and C.
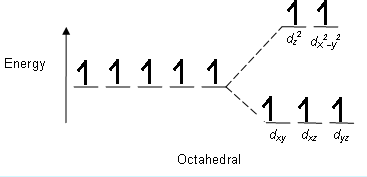
The splitting pattern in K3[FeF6] is;
Atomic number of Fe = 26
Electronic configuration = [Ar]3d64s2
For, Fe+3=[Ar]3d5ort2g3eg2
The crystal field splitting energy for octahedral complexes are given by;
CFSE = −0.4×t2g+0.6×eg
By putting the values, we get
CFSE = −0.4×3+0.6×2
CFSE = 0
Now, for K2[MnF6]
Atomic number of Mn = 25
Electronic configuration = [Ar]3d54s2
For, Mn+3=[Ar]3d4ort2g3eg1
The crystal field splitting energy for octahedral complexes are given by;
CFSE = −0.4×t2g+0.6×eg
By putting the values, we get
CFSE = −0.4×3+0.6×1
CFSE = −0.6Δ0
Hence, we can say that the value for crystal field stabilization energy is zero for K3[FeF6].
The correct option is C.
Note: The possibility to make a mistake is that you may use the crystal field splitting energy formula for tetrahedral, not octahedral. But all the compounds are octahedral so do not confuse between them.