Question
Question: The valency of a chlorine atom is taken as the \(1\), and this value is taken as the standard. A. ...
The valency of a chlorine atom is taken as the 1, and this value is taken as the standard.
A. True
B. False
Solution
The given question clearly states that the valency of the chlorine atom needs to be known and for that the atomic number and valence electron in the chlorine atom need to be stated. The standard value here indicates the value which will not change in any condition that can be explained by giving examples of chlorine bonded with different atoms.
Complete step by step answer:
Chlorine is present in the p-block and is one of the elements from the halogen series. It is placed below the Fluorine atom, the highest electronegative atom in the periodic table. The chlorine has seventeen electrons. Its atomic number is seventeen. The electronic configuration is 1s22s22p63s23p5. The number of electrons in the outer orbital is seven, to form a bond with another atom it can gain an electron, because it will be easy to gain one electron and obtain an octet state with eight electrons in outer orbital, instead of losing seven electrons.
The electronegativity of chlorine is high; it is second after Fluorine atoms hence the number of electrons which will be accepted or shared by the chlorine of another atom will be one. In the given figure, each chlorine will accept one electron from the magnesium atom as magnesium which has specific valency as two, so it will give two electron to each chlorine atom and form Mg+2. Hence,MgCl2 is formed.
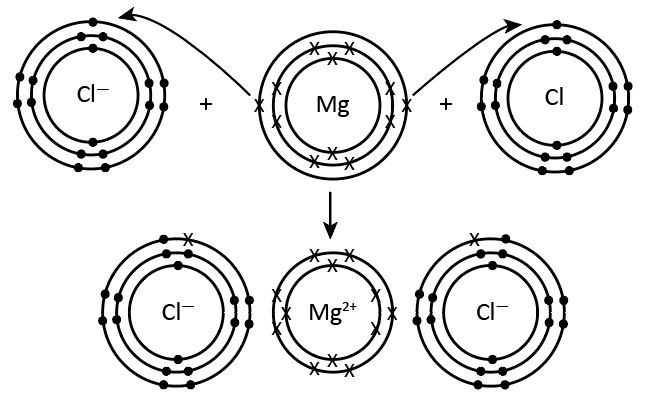
Therefore, we can say the above statement is true chlorine has a specific valency as one.
Note:
The valency of the atom is specific of s-block and p- block while the d-block has a wide range of valency keeps on changing.
