Question
Question: The valencies of nitrogen and boron in borazole are: A. 3, 3 B. 4, 4 C. 3, 4 D. 4, 3...
The valencies of nitrogen and boron in borazole are:
A. 3, 3
B. 4, 4
C. 3, 4
D. 4, 3
Solution
Borazole which is also known as Borazine, is a polar inorganic compound with the chemical formula B3H6N3. It is synthesized from diborane and ammonia in a 1:2 ratio at 250−300∘C with a conversion of 50 %. It is also known as inorganic benzene. The reaction involved in the preparation of borazole is:
3B2H6+6NH3→2B3H6N3+12H2
Complete step by step answer:
The structure of borazole is
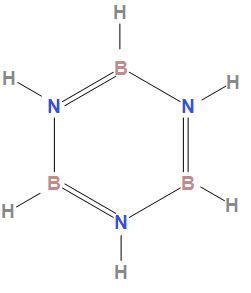
In the structure of borazole we can see that boron forms four bonds and nitrogen also forms 4 bonds due to which their valency is 4
After discussing we can say that the valencies of boron and nitrogen is 4, 4
So, the correct answer is Option B.
Additional Information:
Valency is the measure of the combining capacity of atoms or molecules. Therefore, it is the capacity of an atom of a single element to react and combine with particular numbers of atoms of another element.
Electrons in an atom are arranged in different orbitals. The electrons present in the outermost shell or orbit of an atom are valence electrons.
Note: In borazole the valence shell orbitals are sp2 hybridized. Each nitrogen atom has a one pair of electrons while each boron atom has an empty p- orbital. The bonding in borazole is dative and it arises from the side wise overlapping of fully filled p- orbitals of nitrogen and empty p- orbitals of boron. Because of the similarity of the structures of borazole or borazine and benzene, borazole is also known as inorganic benzene.
Molecular orbital calculations indicate the electrons in borazole are only partially delocalized unlike benzene in which there is a complete delocalization.
