Question
Question: The uranium nucleus \(_{92}{U^{238}}\) emits a \(\alpha \)−particle and the resulting nucleus emits ...
The uranium nucleus 92U238 emits a α−particle and the resulting nucleus emits one β −particle. The atomic number and mass number of the final nucleus will be respectively
A) 91,234
B) 90,234
C) 91,238
D) 92,234
Solution
It is clear that in decay the proton stays in the nucleus but the electron leaves the atom as a beta particle. In alpha decay mass and atomic both will change by subtraction of 4 and 2 whereas in beta decay only mass number increases.
Step by step solution:
Step 1:
The emission of alpha particles is a property of the heaviest nuclei, such as uranium 238 with its 92 protons and 136 neutrons, the heaviest natural nucleus observed. These unstable nuclei emit a light helium nucleus in order to reduce their mass and hence increase their stability.
The proton stays in the nucleus but the electron leaves the atom as a beta particle. When a nucleus emits a beta particle, these changes happen: the mass number stays the same. The atomic number increases by 1.
Step 2:
For more clarity have a look on the diagram:
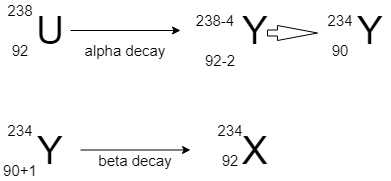
Release of a α will decrease the atomic number by 2 and mass number by 4, and β particle will increase the mass number by 1 without change in mass number.
So final product will have a mass number of 234 and atomic number =91.
Hence option A is correct.
Additional information:
Alpha particles are dangerous: alpha radiation is the most dangerous because it is easily absorbed by cells. Beta and gamma radiation are not as dangerous because they are less likely to be absorbed by a cell and will usually just pass right through it.
Note: All natural uranium isotopes emit alpha particles – positively charged ions identical to the nucleus of a helium atom, with two protons and two neutrons. Their beta and gamma activity is low. Alpha particles are relatively large, and do not penetrate far in tissue – they are stopped by the skin, for example.
