Question
Question: The units of electrochemical equivalent is A.Gram coulomb B.Gram \({{\text{A}}^{ - 1}}{\text{ }}...
The units of electrochemical equivalent is
A.Gram coulomb
B.Gram A−1 s−1
C.Gram A s - 1
D.Gram A - 1 s
Solution
The electrochemical equivalent is measured by a Voltmeter. It is the mass of the substance deposited to one of the electrodes when a current of 1 Ampere is passed for 1 second or the amount of substance deposited by 1 Coulomb. Current is the rate at which electrons flow in a circuit. Ampere is the unit of current. A substance that conducts electricity when dissolved in water is called an electrolyte.
Complete step by step answer:
We have discussed what an electrolyte is. Electrolysis is done when the electricity is passing through an electrolyte.
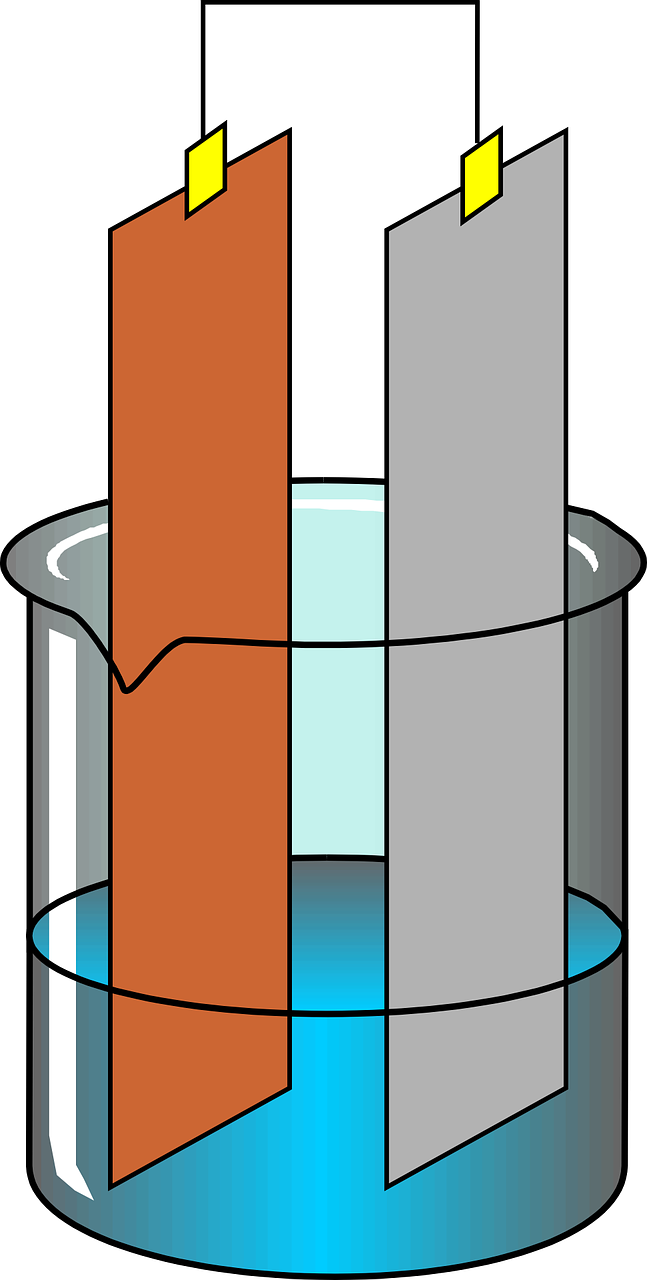
This is how electrolysis takes place. Many chemical reactions are happening during electrolysis.
The positively charged ions from the electrolyte move to the negative electrode and vice versa
Let us now look at Faraday’s first law of electrolysis which is governing this process.
It states that the total mass of a substance deposited at an electrode is directly proportional to the amount of charge passing through the electrolyte.
So we can say that m∝q, where m is the mass of substance deposited on the electrode and q is the charge.
m∝q,
So proportionality is changed, m = z X q
Where z is known as Electrochemical equivalent (ECE).
On rearranging, z = qm, z is the mass deposited on electrode per unit charge and its unit is gram per coulomb. As we already discussed, we know the formula of current as I = tq, I is current, q is the charge and t is the time.
On rearranging, we know that q=It.
Substituting q=It in z = qm, we get z = Itm
Now let us find the unit of z.
Unit of z = unit of ItUnit of mass, we know the unit of I is Ampere and time is sec.
Unit of z is gram A−1 s−1
Thus, the correct option is B.
Note: Electrochemical equivalent is the mass of the substance deposited to one of the electrodes when a current of 1 Ampere is passed for 1 second. It can be also described as the amount of substance deposited by 1 Coulomb. 1 Ampere is 1 Coulomb of electrons moving in 1 second. If the amount of charge flowing through two different systems is the same, then m2m1 = z2z1.
