Question
Question: The unit cell in a body centred cubic lattice is given in the figure. Each sphere has radius \(r\) a...
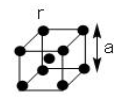
The unit cell in a body centred cubic lattice is given in the figure. Each sphere has radius r and the cube side a. What fraction of the total cube volume is empty?
A) 1−38πa3r3
B) 34πa3r3
C) ar
D) 2−34πa3r3
Solution
To answer this question, you must find the relation between the edge length of the unit cell and radius of the constituent particles. In a body centered cubic unit cell, each corner touches the body centre atom. So, closest contact is along the body diagonal of the cube.
Formula used:
43a=r
Where a is the side of the cube and r is the radius of the constituent atoms.
Complete step by step solution:
In a body centred cubic lattice, the effective number of atoms in the unit cell is n=2 .
Firstly, we must find the volume of the unit cell. We have with us a cubic unit cell.
The volume of a cube is given by
V=a3 ..….
The next step is to find the volume occupied by the atoms.
The volume of a sphere is given by
v=34πr3
Where r is the radius of the sphere.
Hence, we can say that the volume occupied by one atom is v=34πr3
So, the volume occupied by two atoms will bevn=2×34πr3=38πr3 .….
To solve the question further we need to find the relation between the radius of constituent particles and edge length of the unit cell.
The body diagonal of the cube is given by d=3a
The body diagonal of the unit cell is equal to 4 times the radius of constituent atoms.
Hence,
⇒3a=4r
⇒43a=r ……
Substituting the value of r in terms of a into equation (2), we get
vn=38π(43a)3
vn=83πa3 …..
Volume of cube that is empty is given by,
ve=V−vn=(1−83πa3)≈0.3a3
Fraction of the total cube volume empty:
V−vnve=a30.3a3=0.3
Note:
To find the number of atoms in a unit cell we use the formula
n=8nc+2nf+1ni+4ne
where,n is effective number of atoms in unit cell
nc is number of atoms at the corners of the unit cell
nf is number of atoms at the six faces of the unit cell
ni is number of atoms completely inside the unit cell
ne is number of atoms at the edge centres of the unit cell
Firstly, we start by finding the effective number of atoms in the unit cell
In a body centred cubic unit cell, atoms are present at the corners and the body centre of the cube. So, we can write that,
nc= number of atoms at the corners of the unit cell =8
nf=number of atoms at the six faces of the unit cell =0
ni= number of atoms completely inside the unit cell =1
ne=number of atoms at the edge centres of the unit cell =0
Thus, the total number of atoms in a body centred unit cell is
n=8nc+2nf+1ni+4ne
Substituting the values, we get
n=88+20+11+40
n=1+0+1+0
Thus, n=2
