Question
Question: The types of hybrid orbitals of nitrogen in \[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\] respec...
The types of hybrid orbitals of nitrogen in NO2+, NO3− and NH4+ respectively are expected to be:
A. sp, sp3 and sp2
B. sp, sp2 and sp3
C. sp2, sp and sp3
D. sp2, sp3 and sp
Solution
To find the types of hybrid orbitals involved in hybridization there is a formula. It is as follows.
H=21[VC+A]
Where H is the number of orbitals involved in hybridization
V is the number of valence electrons of central atom
M is the number of monovalent atoms attached to central atom
C = charge on the cation
A = charge on the anion
Complete step by step answer:
- The given molecules areNO2+, NO3− and NH4+.
- The structure of NO2+ is as follows.
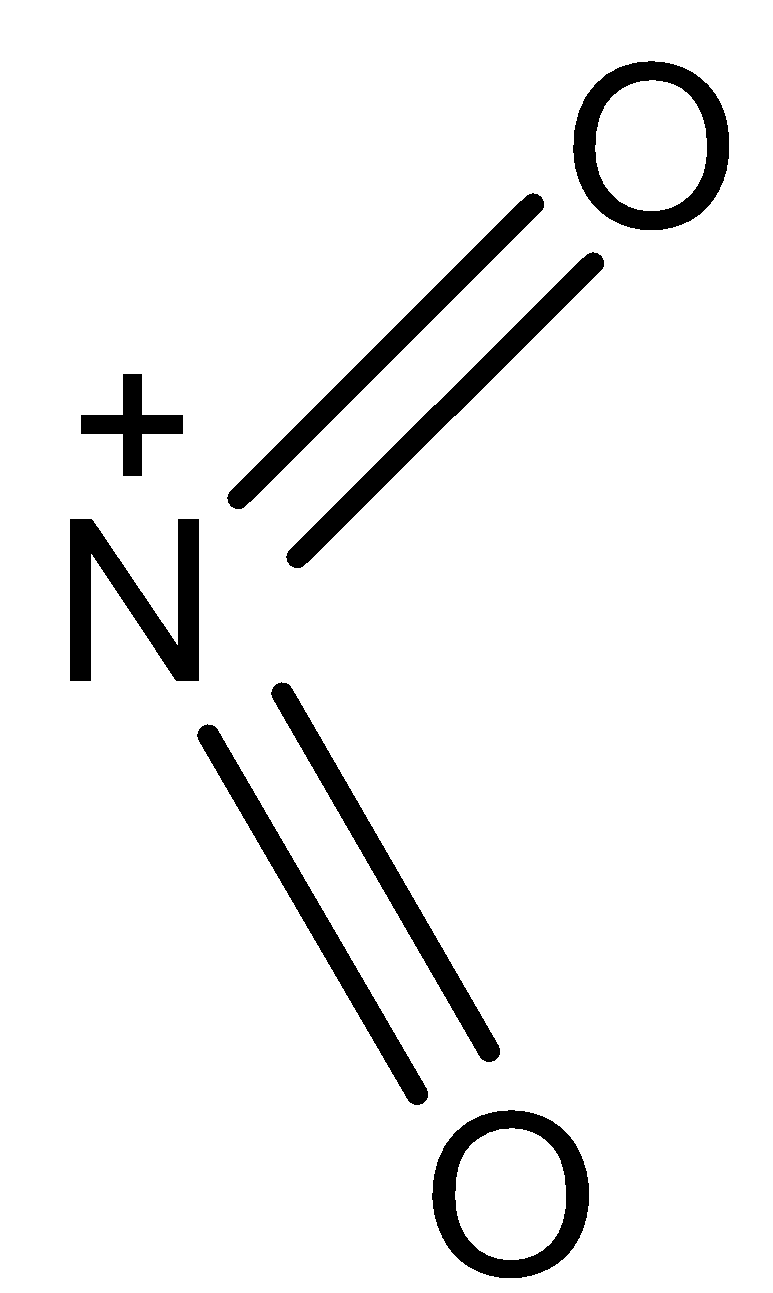
-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 0
C = charge on the cation = 1
A = charge on the anion = 0
- Therefore
