Question
Question: The type of ion found in potassium phosphates is: [A] \({{X}^{+}}\) [B] \({{X}^{2+}}\) [C] \({...
The type of ion found in potassium phosphates is:
[A] X+
[B] X2+
[C] X3+
[D] XO32−
[E] XO43−
Solution
This question has multiple correct answers. One answer will be for the cation and the other for anion. A phosphate ion consists of 4 oxygen atoms, one forms a double bond with phosphorus whereas the others are negative ions.
Complete answer:
We use the term potassium phosphate to describe some general forms of salts formed by potassium and phosphate ions.
We know that phosphate ion is PO43−. One oxygen atom forms a double bond with the potassium atom and the other oxygen atoms stay as negatively charged ions thus, it has a negative charge of 3. We can draw the structure of phosphate as ion as-
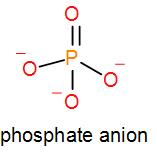
Phosphate combined with potassium gives us 3 kinds of compounds namely mono potassium phosphate, di-potassium phosphate and tripotassium phosphate.
As we can understand from their names itself monophosphate contains one potassium ion whereas di- and tri- potassium phosphates will contain 2 and 3 potassium ions respectively.
In mono potassium phosphate, di-potassium phosphate and tripotassium phosphate respectively one, two and three hydrogen atoms are replaced by potassium ions. Now we can draw their structures as-
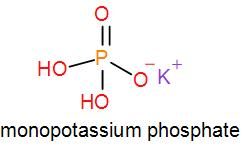
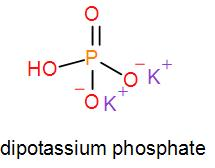
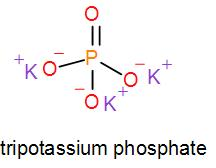
As we can see from the above structures that a potassium cation is found in all the structures therefore, the type of cation found in potassium phosphates is K+ which is in the form X+. Therefore, option [A] is one of the correct answers.
Similarly, we can see that it has phosphate ions which is PO43− and it is of the type XO43−. So, option [D] is also a correct option.
Therefore, the correct answer is option [A] X+ and option [D] XO43−.
So, the correct answer is “Option A and D”.
Note: We use mono-potassium phosphate together with di-potassium phosphate, a fertilizer, food additive and also as a buffering agent. Tri-potassium phosphate is used as a strong base. Phosphates salts are also used in the medicine industry.
