Question
Question: The type of hybrid orbitals used by chlorine atom in \(Cl{O_2}\) is: (A) \(s{p^3}\) (B) \(s{p^2}...
The type of hybrid orbitals used by chlorine atom in ClO2 is:
(A) sp3
(B) sp2
(C) sp
(D) sp3d
Solution
Hybridisation is the data that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridisation is also an expansion of the valence bond theory.
Complete step by step answer:
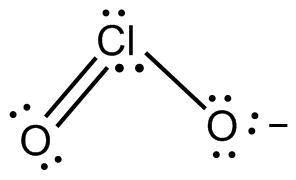
We know that ClO2− is having the sp3 hybridization.
In the ground state electronic configuration of Cl is 3s23p5 and the excited electron configuration of Cl is 3s23p43d1. So, the electronic configuration of O will be 2s22p4
From above we get that the chlorine is having sp3 hybridization. The lone electrons are in s and p orbitals while the two singly filled p – orbitals will be forming the sigma bonds. While the pi bond is being formed after that by the overlap of the half filled 3d orbitals with the half filled 2p orbital of the O atom.
So, the answer is (A) sp3hybridization and hybrid orbitals.
Additional information:
Uses of ClO2:
1. It is used as disinfectant in treatment of water.
2. Used as antimicrobial agents in water that are used in processing poultry, washing vegetables and fruits.
3. Used for processing wood pulp that is used for making paper.
4. Used for sterilizing medical equipment.
Note:
ClO2− has tetrahedral geometry and molecular geometry is bent. Since there are 2 lone pairs on chlorine the electron pair repulsion will result in a bond angle that will be slightly less than 109.5∘.
