Question
Chemistry Question on Chemical bonding and molecular structure
The type of bonds present in sulphuric anhydride are
A
3σ and three pπ−dπ
B
3σ, one pπ−dπ and two pπ−dπ
C
2σ and three pπ−dπ
D
2σ and two pπ−dπ
Answer
3σ, one pπ−dπ and two pπ−dπ
Explanation
Solution
Sulphuric anhydride is SO3 and its structure is as follows:
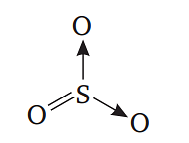
⇒3σ,1pπ−pπ,2pπ−dπ bonds are present.
