Question
Question: The type of attractive forces between a polar molecule and a non-polar molecule are...
The type of attractive forces between a polar molecule and a non-polar molecule are
A
Dipole-dipole forces
B
Hydrogen bonds
C
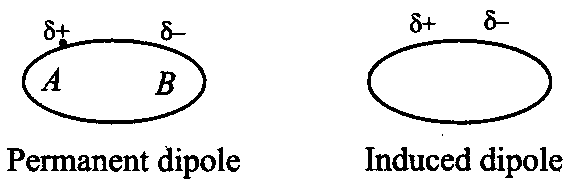
Dipole-induced dipole forces
D
Dispersion forces.
Answer
Dipole-induced dipole forces
Explanation
Solution
: It is the type of force between the polar molecule and a non-polar molecule. Dipole of polar molecule induces dipole on the electrically neutral molecule.