Question
Question: The two monomers for the synthesis of Nylon 6, 6 are: (a)- \(HOOC{{(C{{H}_{2}})}_{6}}COOH,\text{ }...
The two monomers for the synthesis of Nylon 6, 6 are:
(a)- HOOC(CH2)6COOH, H2N(CH2)6NH2
(b)- HOOC(CH2)6COOH, H2N(CH2)4NH2
(c)- HOOC(CH2)6COOH, H2N(CH2)4NH2
(d)- HOOC(CH2)4COOH, H2N(CH2)6NH2
Solution
Nylon 6,6 is a polymer that is formed by condensation polymerization. It is made up of two monomers. The 6,6 in Nylon 6,6 indicates that both the monomers of Nylon 6,6 have 6 carbon atoms each.
Complete step by step answer:
Nylon 6,6 is a polymer. It comes under the polymers which have amide linkage. Amide linkage is formed between the carboxyl group of one molecule and the ammonia group of another molecule. The linkage is −NH−CO−. The linkage is caused by the elimination of a water molecule.
Nylon 6,6 is a polyamide. It comes under condensation polymerization because there is a loss of water molecules during the polymerization. There are two monomers in Nylon 6,6. These monomers are adipic acid and hexamethylenediamine. The formula of adipic acid is HOOC(CH2)4COOH. It is made up of 6 carbon atoms. Each terminal is a carboxylic group. The formula of hexamethylenediamine is H2N(CH2)6NH2. It is also a 6 carbon atom molecule. Each terminal carbon atom is attached to the amine group.
The acid and the amine are heated at 525 K under pressure and undergo polymerization with the elimination of water as steam, nylon 6,6 is produced. The reaction of formation of Nylon 6,6 is given below:
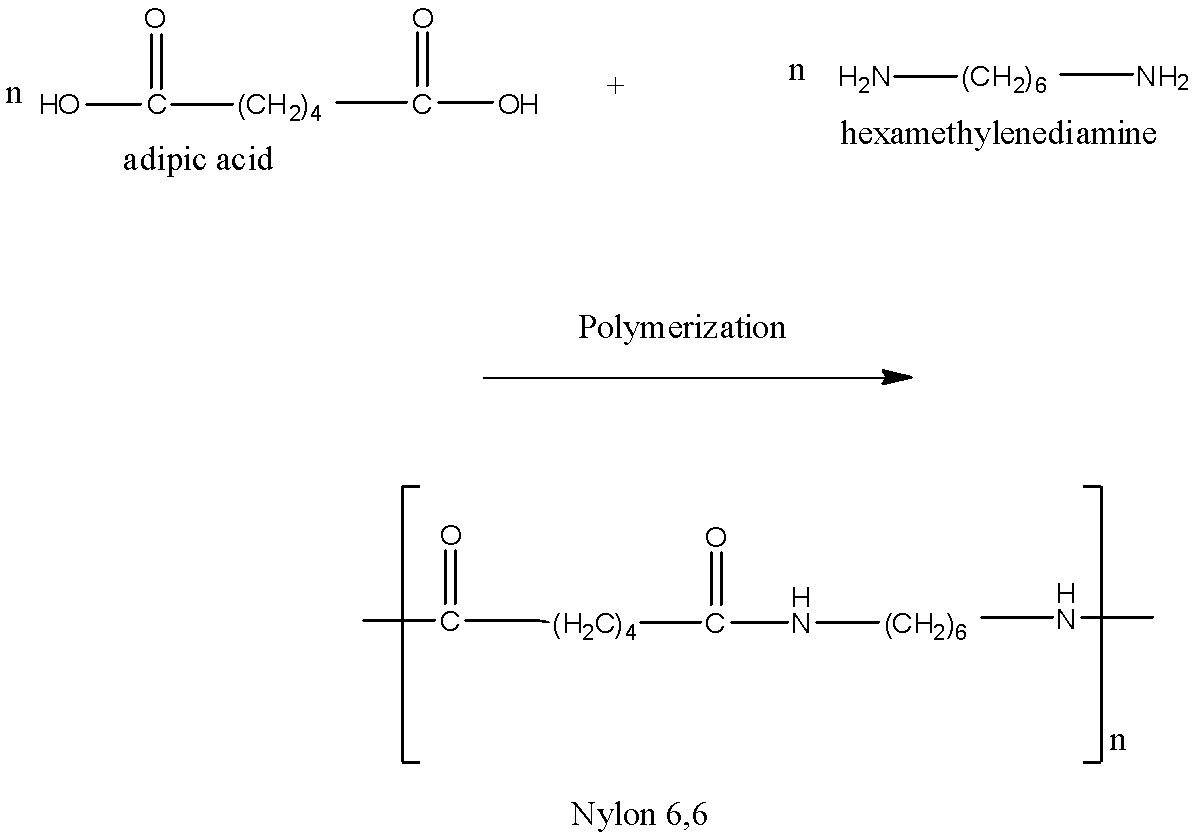
Since both the monomers have 6 carbon atoms each, therefore, it is written as Nylon 6,6.
So, the correct answer is - HOOC(CH2)4COOH, H2N(CH2)6NH2.
So, the correct answer is “Option D”.
Note: There are two more Nylon produced by condensation reaction:
(i)- Nylon 6,10, whose monomers are sebacic acid HOOC(CH2)8COOH and hexamethylenediamine H2N(CH2)6NH2
(ii)- Nylon 6, whose monomer is 6-Aminohexanoic acid H3N+−(CH2)5−COO−.
