Question
Question: The two functional groups present in a typical carbohydrate are: A. –OH and –COOH B. –CHO and –C...
The two functional groups present in a typical carbohydrate are:
A. –OH and –COOH
B. –CHO and –COOH
C. > C=O and –OH
D. –OH and –CHO
Solution
Carbohydrates are the biomolecules made up of carbon, hydrogen and oxygen. The general formula of carbohydrate is (Cn(H2O)m) . The examples of the carbohydrates are glucose, fructose, starch, cellulose and etc.
Complete step by step answer:
- The general definition is carbohydrates are polyhydroxy aldehyde or ketones and they are optically active due to the presence of chiral centers in their structures.
- We have to check all the options to know which one is correct as per the above definition.
- Coming to option A, –OH and –COOH. As per the above definition carbohydrates contain hydroxyl, aldehyde or ketone in their structure. but in the option the carboxyl group is present. so, it is wrong.
- Coming to option B, –CHO and –COOH. It is also because carbohydrates contain aldehyde functional groups but not carboxylic acid functional groups in their structure.
- Coming to option C, > C=O and –OH. It is correct because the given functional groups are ketone and hydroxyl, as per the definition carbohydrates contain ketone and hydroxyl groups in their structure.
- Coming to option D, –OH and –CHO. It is also correct because the given functional groups are aldehyde and hydroxyl, as per the definition carbohydrates contain aldehyde and hydroxyl groups in their structure.
- Therefore the correct options are C and D.
Note: The best example for carbohydrate is glucose. The structure of the glucose is as follows.
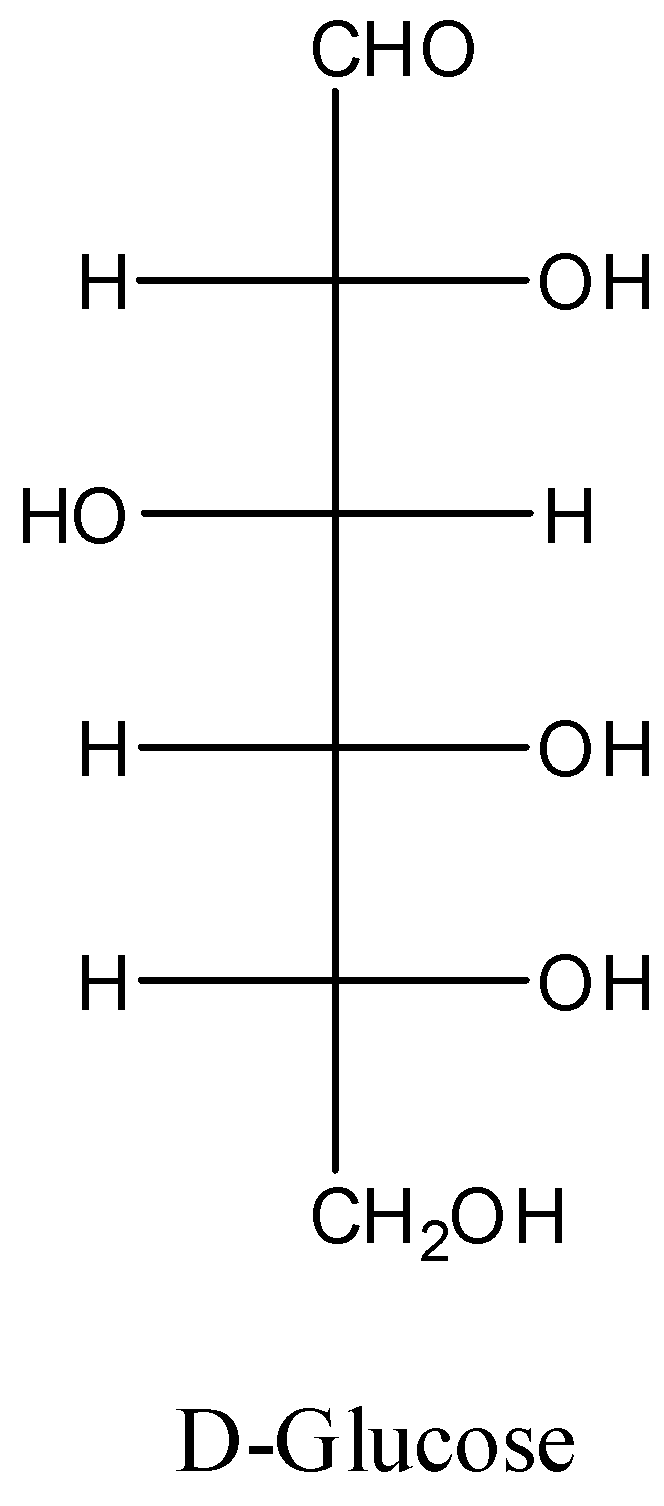
We can see there are five hydroxyl groups (-OH) in its structure and there is one aldehyde functional group also.
