Question
Question: The treatment of alcohol of alkyl chlorides with aqueous \(KOH\) leads to the formation of alcohols ...
The treatment of alcohol of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Solution
When alkyl chlorides are treated with aqueous KOH the KOH releases its. OH− ion only. This ion acts as a strong nucleophile. Due to this causes hydrolysis of alkyl chloride to their corresponding alcohols . Substitution reaction takes place.
RCL+OH−hydrolysisROH+Cl−
Complete step by step answer: Now when KOH is alcoholic is doesn’t have OH−ion rather it has ethoxide ionC2H5O−. This ion is more basic than hydroxide ions. The ethoxide ion causes dehydrohalogenation to form alkenes. The ethoxide ion abstracts the beta hydrogen from alkyl chloride. Now a molecule of KCl gets eliminated and alkene is formed. This reaction is an elimination reaction.
When alkyl chloride reacts with aqueous KOH
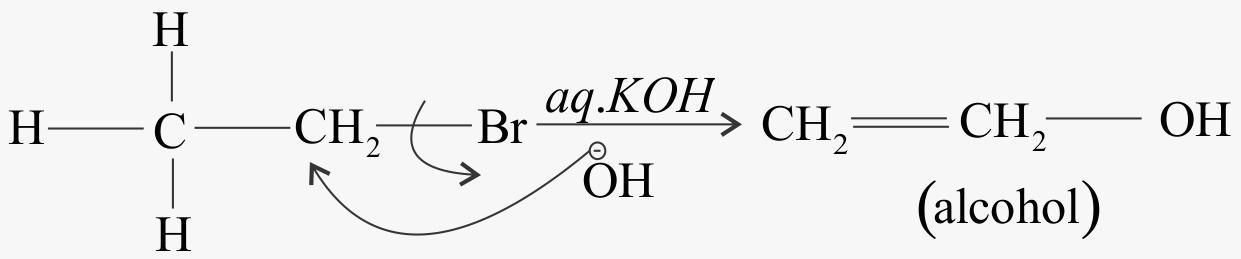
When alkyl chloride reacts with alcoholic KOH
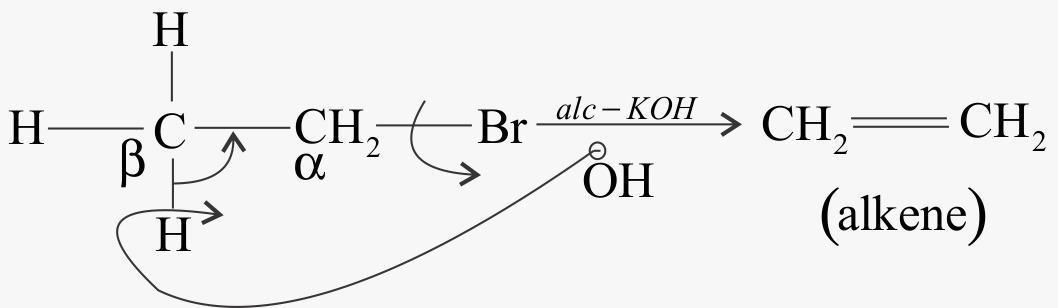
The type of elimination is beta elimination.
CH3−CHCl−CH3+KOH→CH3−CH≐CH2+KCl+H2O
It is a single step bimolecular elimination reaction
Note: Haloalkanes having two or more types of beta hydrogen atoms on dehydrohalogenation gives more than one alkene. Major product is decided by Saytzeff’s rule which states that haloalkanes on heating with alcoholic KOH give more substituted alkene as a major product.
When a bulky base like propoxide ion is used then less substituted alkene is the major product because bulky groups find it easier to remove protons from less sterically hindered beta carbon atoms.
