Question
Question: The trans-alkenes are formed by the reduction of alkynes with A.\[Na/liq.{\text{ }}N{H_3}\;\] B...
The trans-alkenes are formed by the reduction of alkynes with
A.Na/liq. NH3
B.Sn−HCI
C.H2−Pd/C ,BaSO4
D.NaBH4
Solution
To answer this question recall the methods for the preparation of alkyne from dihalides. Sodium amide is a strong reducing agent which removes the halide atoms and generates an alkyne.
Complete step by step answer:
Taking a look at the options:
Sodium metal in the presence of ammonia will reduce alkyne through trans addition leading to formation of trans alkenes. The atom of sodium donates an electron. This is followed by the removal of H from ammonia and its addition on alkyne which forms a vinylic anion. This vinylic anion is much more stable in the trans form compared to the cis form. This reaction is named as Birch reduction.
Sn−HCI: This option is incorrect and can be eliminated
H2−Pd/C ,BaSO4: This option is incorrect and can be eliminated
NaBH4: This option is incorrect and can be eliminated
Sodium amide (NaNH2) is a strong base and is used for deprotonation of weak acids and also for elimination reactions. Treatment of either geminal dihalide (two halogens on one carbon) or vicinal dihalides (halogens on adjacent carbons) with two equivalents of NaNH2 results in the formation of alkynes. The mechanism of this reaction can be shown as:
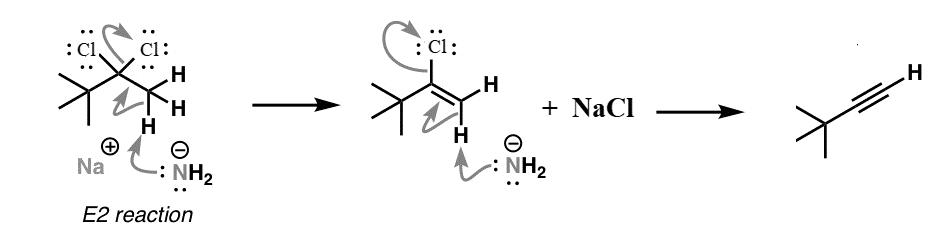
We can see from the above reaction that we receive an alkyne as the major product of the reaction.
Hence, the correct option is option A.
Note:
The acidity of terminal alkynes plays an important role in major product determination when dihalides undergo base induced elimination reactions. High electronegativity of the triple bond in terminal alkynes makes the molecule acidic. Therefore, one of the base molecules will pull off the terminal hydrogen instead of one of the halides like we desire to happen in this reaction. This implies that we would need three bases for every terminal haloalkane instead of two to obtain an alkyne.
