Question
Question: The total number(s) of stable conformers with non-zero dipole moment for the following compound is (...
The total number(s) of stable conformers with non-zero dipole moment for the following compound is (are):
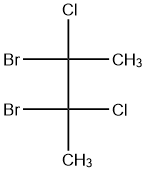
(A) 1
(B) 2
(C) 3
(D) none of these
Solution
Any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the energy surface are specifically called conformational isomers or conformers.
Complete step by step answer:
Conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation).
A dipole moment arises in any system in which there is a separation of charge. They can, therefore, arise in ionic bonds as well as in covalent bonds. Dipole moments occur due to the difference in electronegativity between two chemically bonded atoms.
The three conformers of the compound given are as follows:
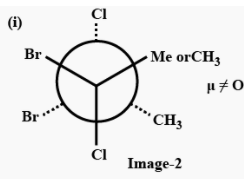
In (i), Chlorine atoms are present trans to each other so there will be no dipole moment because of chlorine but bromine atoms are present cis to each other, so there will be a net dipole moment, because of bromine atoms, therefore conformer (i) has a non zero dipole moment.
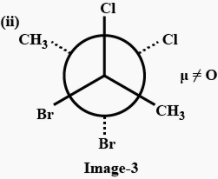
In (ii), Chlorine atoms are present cis to each other, and bromine atoms are present cis to each other, so both of them will generate dipole moment, and since chlorine is more electronegative so the net dipole moment will be directed towards the chlorine atom. Therefore conformer (ii) has a non-zero dipole moment.
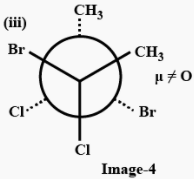
In (i), bromine atoms are present trans to each other so there will be no dipole moment because of bromine but chlorine atoms are present cis to each other, so there will be a net dipole moment, because of chlorine atoms, therefore conformer (i) has a non zero dipole moment.
So, the correct answer is “Option C”.
Note: A bond dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule. It involves the concept of electric dipole moment, which is a measure of the separation of negative and positive charges in a system.
