Question
Question: The total number of \[\sigma \] and \[\pi \] bonds present in azobenzene are: A.24 \[\sigma \] , 6...
The total number of σ and π bonds present in azobenzene are:
A.24 σ , 6 π
B.25 σ, 7 π
C.28 σ, 6 π
D.27 σ, 6 π
Solution
To obtain the solution of this question, we must first understand the concept of σ bonds and π bonds. Then we must draw the molecular structure of azobenzene and then individually count σ bonds and π bonds.
Complete step by step answer:
Before proceeding with the solution of this question, let us understand some basic concepts and terms:
σ and π bonds are both covalent in nature
σ bonds are formed when there is end to end overlapping of orbitals. It can also be represented as a single bond
π bonds are formed when there is overlapping of a lobe of one atomic orbital. It can also be represented as a double bond.
The bond strength of σ bonds is stronger than π bonds
The structure of Azobenzene can be given by:
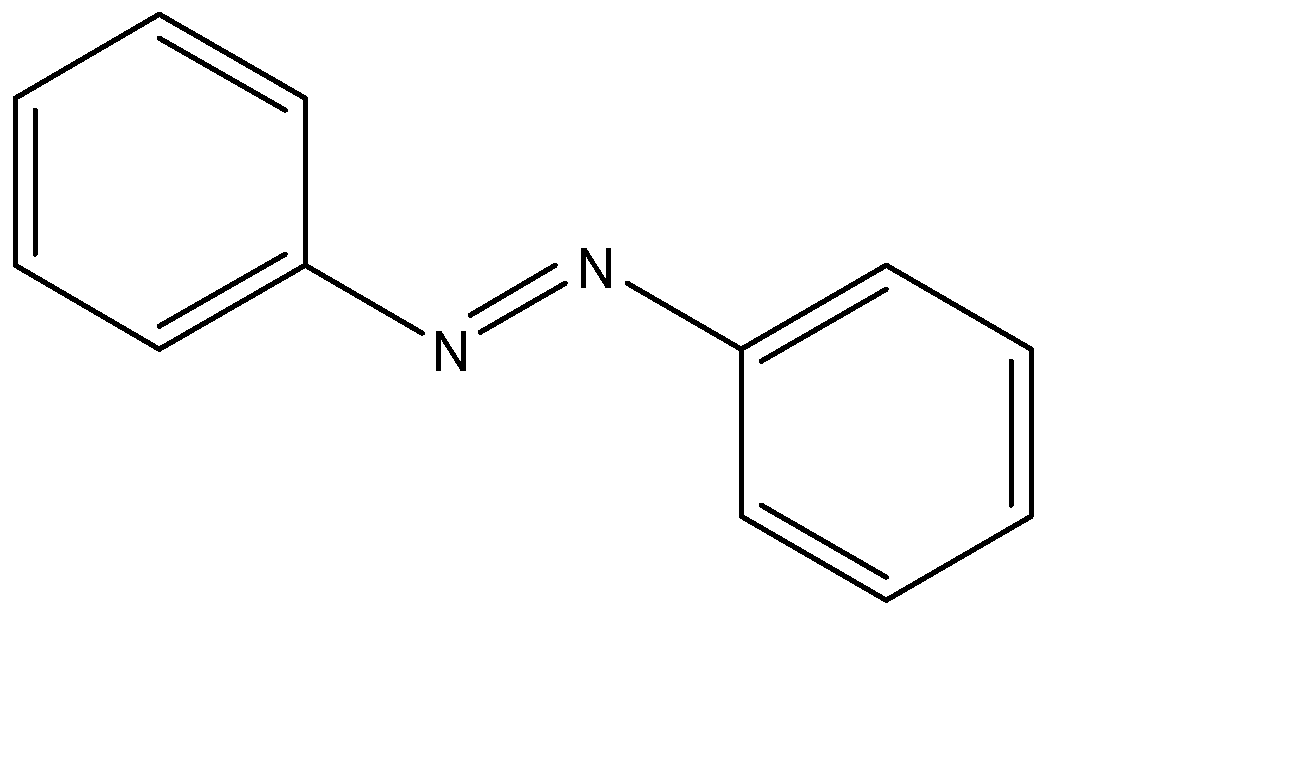
From this structure, we can observe that the number of single and double bonds are:
Number of single bonds = 25 = number of σ bonds
Number of double bonds = 7 = number of π bonds
Hence, there are 25 σ bonds and 7 π bonds
Hence, Option B is the correct option.
Note:
Azobenzene is a molecule whose structure comprises two phenyl rings linked by a N=N double bond; the parent compound of the azobenzene class of compounds. One of the largest applications of the compound azobenzene takes place in the colouring and dye industry.
