Question
Question: The total number of \(\pi \) electrons in the given structure is: 
(a)- 12
(b)- 16
(c)- 4
(d)- 8
Solution
In a compound, there are two types of bonds: sigma and pi bond. The Sigma bond is represented with a single bond and the pi bond is a double and triple bond. Each bond has two electrons.
Complete answer:
In an organic compound, the bonds are mostly made up of two bonds: a sigma bond and a pi bond. If the compound has a single bond then, the compound is having only a sigma bond. If the compound is having a double bond then in the compound one bond is the sigma bond and one bond is the pi bond. If the compound is having a triple bond, then in the compound one bond is a sigma bond and the other two bonds are pi bonds. The electrons of the sigma bond are known as sigma electrons and the electrons of the pi bond are known as pi electrons.
So, in the compound given:
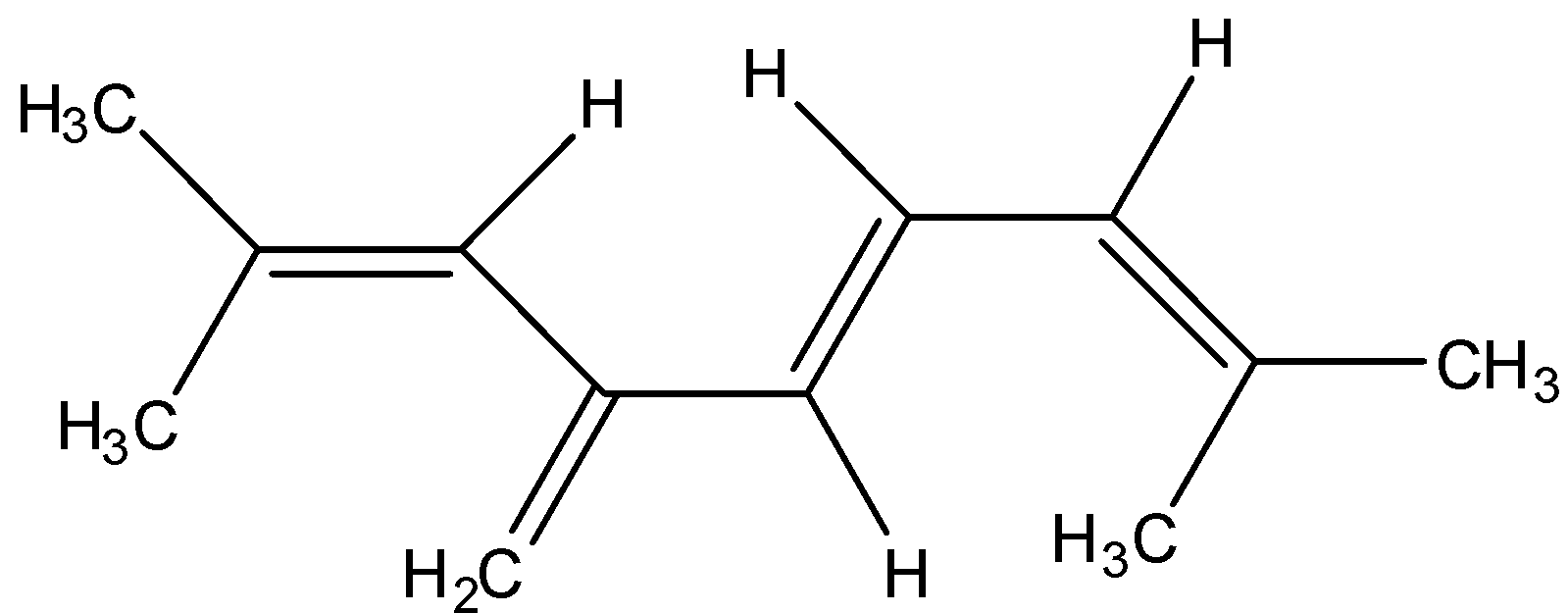
The compound is known as tetraene because there are 4 double bonds. So we can say that there are four pi bonds in this structure. And we know that there are 2 electrons in one bond. And this compound has 24 sigma bonds.
So, there are:
4 x 2 = 8
8 π-electrons in this compound.
And:
28 x 2 = 56
56 σ−electronsin this compound.
Therefore, the correct answer is an option (d)- 8.
Note:
If the compound is having a triple bond then it has two sigma electrons and four pi electrons. These bonds are only formed if the compound has a covalent bond.
