Question
Question: The total number of lone-pairs of electrons in melamine is:...
The total number of lone-pairs of electrons in melamine is:
Solution
Hint: These questions can be solved if you know the structure of the following given compound and the catenation power of the elements forming the given compound and you should know about the valency of the elements to determine whether it will gain electrons or lose electrons to complete its octet.
Complete step by step solution:
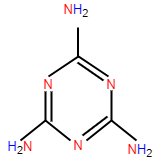
A lone pair is that pair of electrons which are not shared in another atom by covalent bond .They are found in the outermost shell of the atom. In other words, lone pairs are simply the pairs of electrons which do not participate in bond formation or bonding. They can also be called an unshared pair of electrons. If we have to identify these lone pairs we can simply use Lewis dot structure. According to the question we are asked to find out the total number of lone pairs in melamine, so let us first discuss melamine and its structure. Melamine has molecular formula C3H6N6 or C3N3(NH2)3 , and its IUPAC naming is 1,3,5-triazine
The structure is as follows:
As we know, nitrogen has 5 electrons in its outermost valence shell and hydrogen has 1 electron in its outermost valence shell and carbon has 4 electrons in its outermost shell. As we can see in the above structure of melamine nitrogen has bonding with both carbon and hydrogen. As nitrogen has shared its three electrons out of 5 electrons with one carbon and two hydrogen atoms , which means nitrogen has 2 electrons left in its outermost shell which are not shared by any other atom. From this conclusion we can say that now nitrogen has only one pair of electrons left in its outermost shell and as we have discussed above about lone pairs which are those pairs that do not participate in bonding. Thus, we can say that nitrogen has 1 lone pair left. Now, in the structure above we can see that there are 6 nitrogen which means it will form a bond with hydrogen and carbon with 3 bonds , similar as we have discussed before.
Hence, we can say that nitrogen has a total 6 lone pairs of electrons.
Note: We can prepare melamine by breaking down urea into cyanuric acid which then can be reacted to form melamine. Melamine can be used in industrial uses, as glues, as dinnerware, and as flame retardants. In daily life melamine is added to milk to increase the nitrogen content in milk and consequently increasing the protein content.