Question
Question: The total number of isomers of \[{{C}_{4}}{{H}_{8}}\]. A.5 B.6 C.7 D.8...
The total number of isomers of C4H8.
A.5
B.6
C.7
D.8
Solution
In the above question we are asked about the number of isomers of C4H8. Isomers are classified based on various structures and are named accordingly. Like there are position isomers, chain isomers, functional group isomers etc.
Complete step by step solution:
Molecules with different structures but having different structures are known as isomers. Positional isomers are constitutional isomers that have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on or in the carbon chain.
The structures which have different functional groups but have the same molecular formula are known as functional isomers.
The structures which have different structures of carbon atoms but have the same molecular formula are known as Chain isomers.
We are given the structure of C4H8 which is butene or but-1-ene.
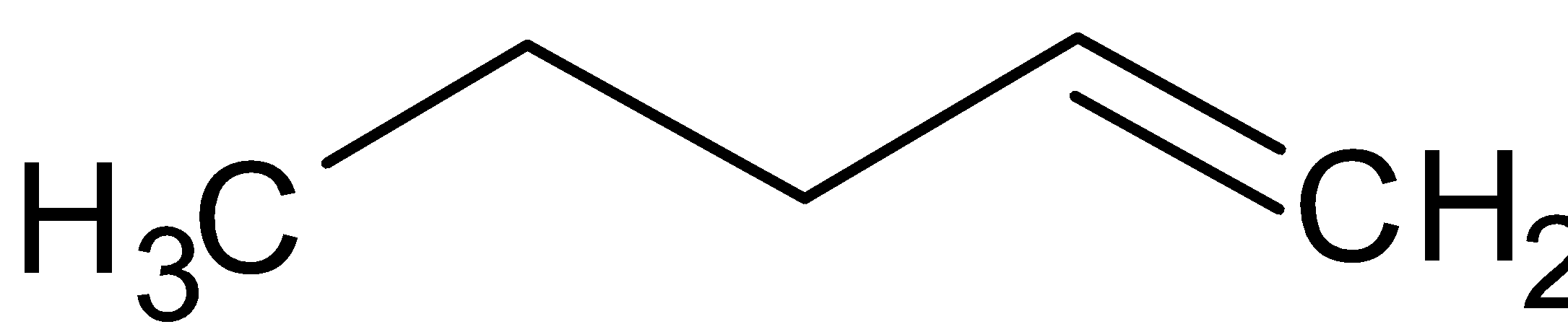
We can shift the bond and get but-2-ene.
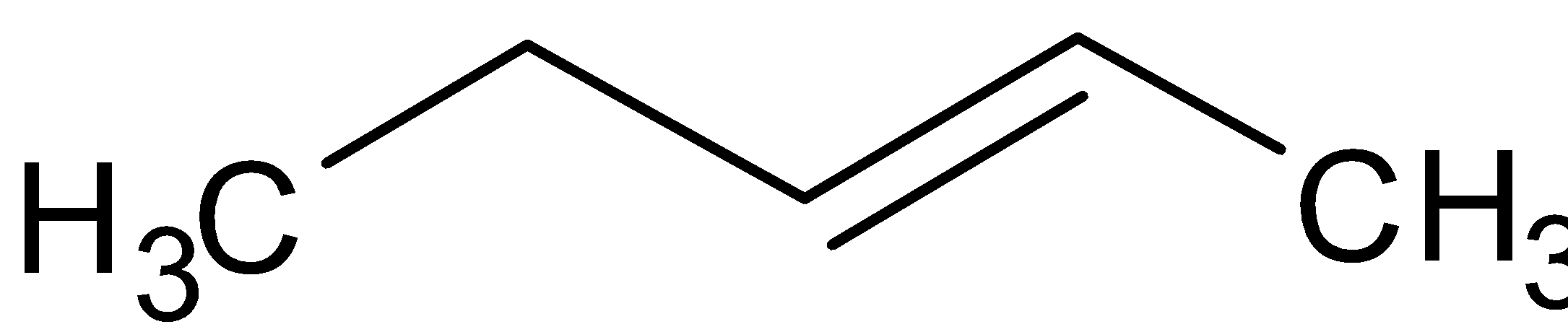
We can shift the methyl group in the secondary chain and get tertiary butane. The structure is as follows:
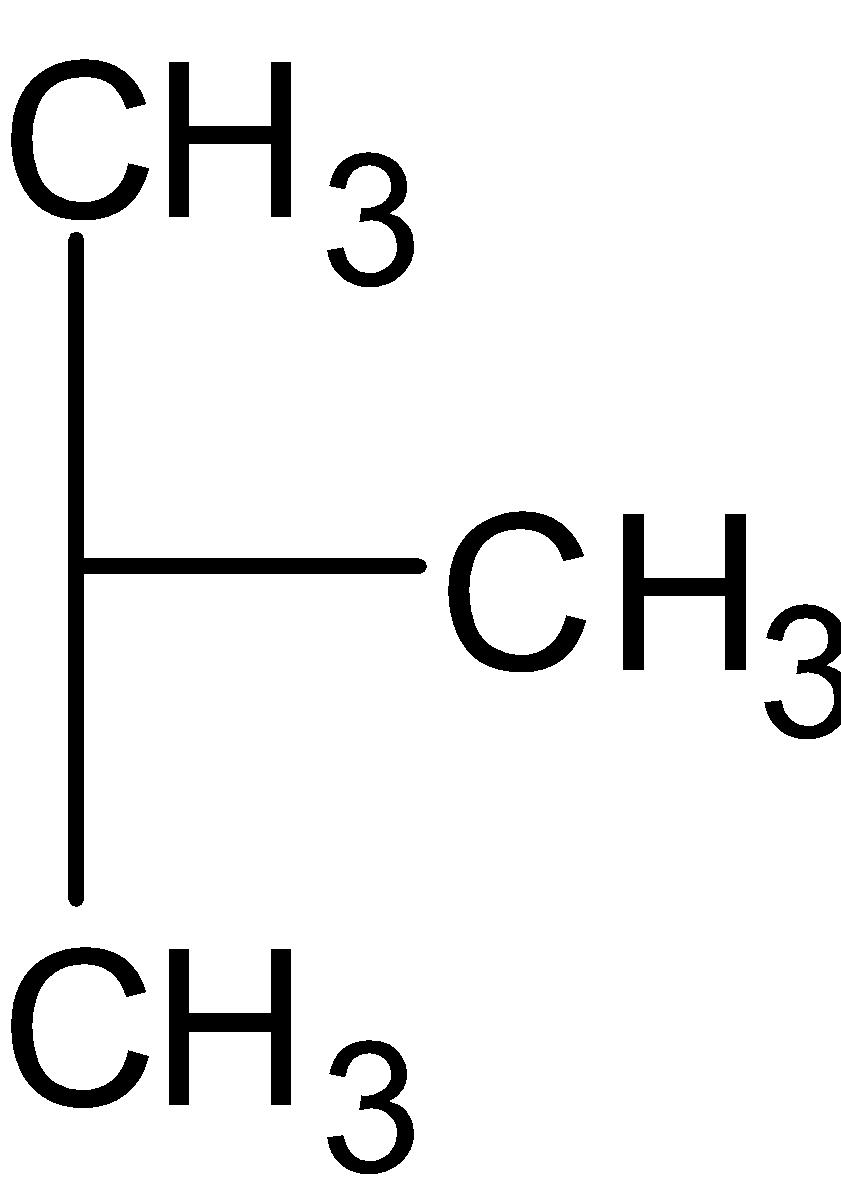
We can make a cyclic structure with the same molecular formula. Then we get cyclobutane. The structure is follows:

Another cycle can be made using propane and we can add a methyl group to get the same molecular formula.
The structure is as follows:
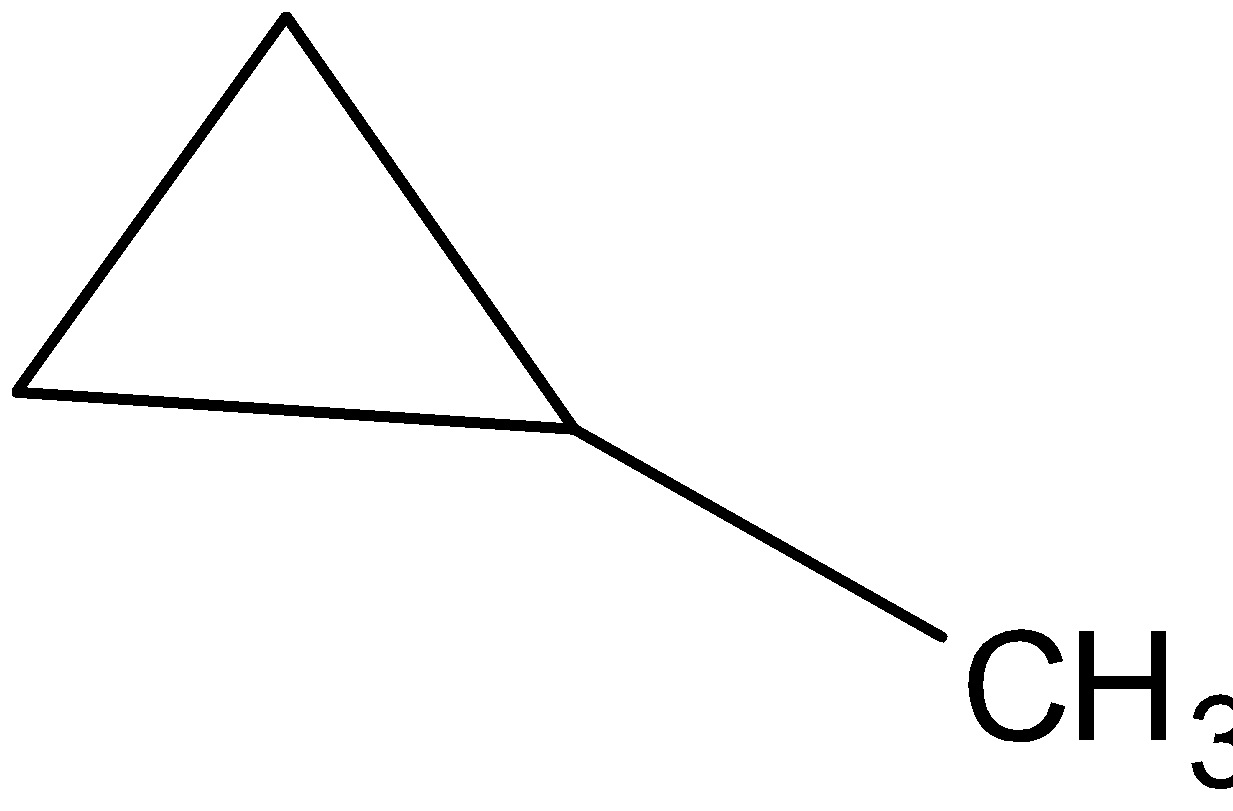
No more structure can be made using this molecular formula. So only 5 different structures are possible which have the same molecular formula.
So the correct option is A.
Note: For solving this type of question we need to draw all the possible structures with the given molecular formula. We then need to count the number of structures and find the answer. It should be noted that if one structure is not calculated then we will get a wrong answer.
