Question
Question: The total number of electrons that take part in forming the bond \({N_2}\) is: A.2 B.4 C.6 D...
The total number of electrons that take part in forming the bond N2 is:
A.2
B.4
C.6
D.10
Solution
We will draw the electron sharing between N atoms by Lewis Structure. Nitrogen N is present in Group 5A, so the number of electrons in its valence shell is 5.Atomic number of Nitrogen is 7.
Complete step by step answer:
Nitrogen is a colorless and odorless gas which is present in the atmosphere and contributes for around 78%. It is used in food processing, in purging air conditioning and refrigeration systems, and in pressurizing aircraft tires.
According to the Lewis Structure in N2 a covalent nonpolar bond is formed between nitrogen atoms with an electronic configuration of 2,5 as its atomic number is 7.
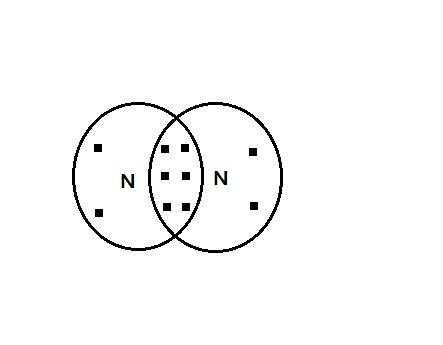
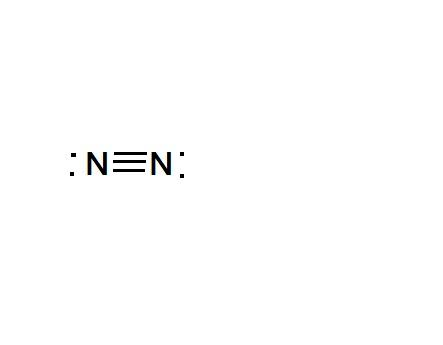
As the figure shows there are six electrons shared between Nitrogen atoms, indicated by 3 pi bonds.
Hence the correct answer is option C.
Additional information- Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule but at the same time. It could not explain the release of energy during the structure of a covalent bond and the shapes of molecules, which can be noted as its limitation.
Note:
N2 are inorganic compounds of nitrogen where it has an oxidation state of -3
Molecular Mass of N2 is 28.0134 g/mol. It was first discovered by Daniel Rutherford in 1772. Nitrogen cannot exist as single atom N, it is always make bond with other N atom and forms N2
Lewis structures are very useful to predict whether ionic or covalent bonds will form between certain elements
