Question
Question: The total number of diprotic acids among the following is \({{H}_{3}}P{{O}_{4}},{{H}_{2}}S{{O}_{4...
The total number of diprotic acids among the following is
H3PO4,H2SO4,H3PO3,H2CO3,H2S2O7,H3BO3,H3PO2,H2CrO4,H2SO3
(A) 6
(B) 5
(C) 4
(D) 2
Solution
Acid continuing oxygen, those compounds are known as oxoacids of oxyacids. More specifically, these oxoacids are containing oxygen and contain at least another element. Oxoacids have at least one hydrogen atom bonded to oxygen and in which forms ions in solution by loss of one more proton.
Complete answer:
The general formula of oxoacids is HxEOy , where E is a non-metal or transition metal and oxygen is directly attached to acidic hydrogen. The determining factor of oxoacid's relative strength is based on the electronegativity of the atom and the number of oxygen atoms around the central atom.
Oxoacids, which have two acidic protons attached to oxygen are known as diprotic acids.
Phosphoric acid - H3PO4
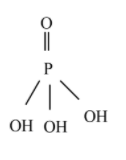
From the above structure, three acidic protons are attached to the oxygen atom. Hence, phosphoric acid is triprotic acid.
Orthophosphoric acid - H3PO3
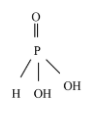
From the above structure, two acidic protons are attached to two oxygen atoms. Hence, Orthophosphorous acid (H3PO3) is a diprotic acid.
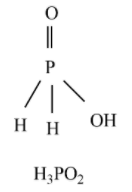
Hypophosphorous acid - H3PO2 is a monoprotic acid
Structures of given oxoacids of sulfur - H2SO3,H2SO4,H2S2O7
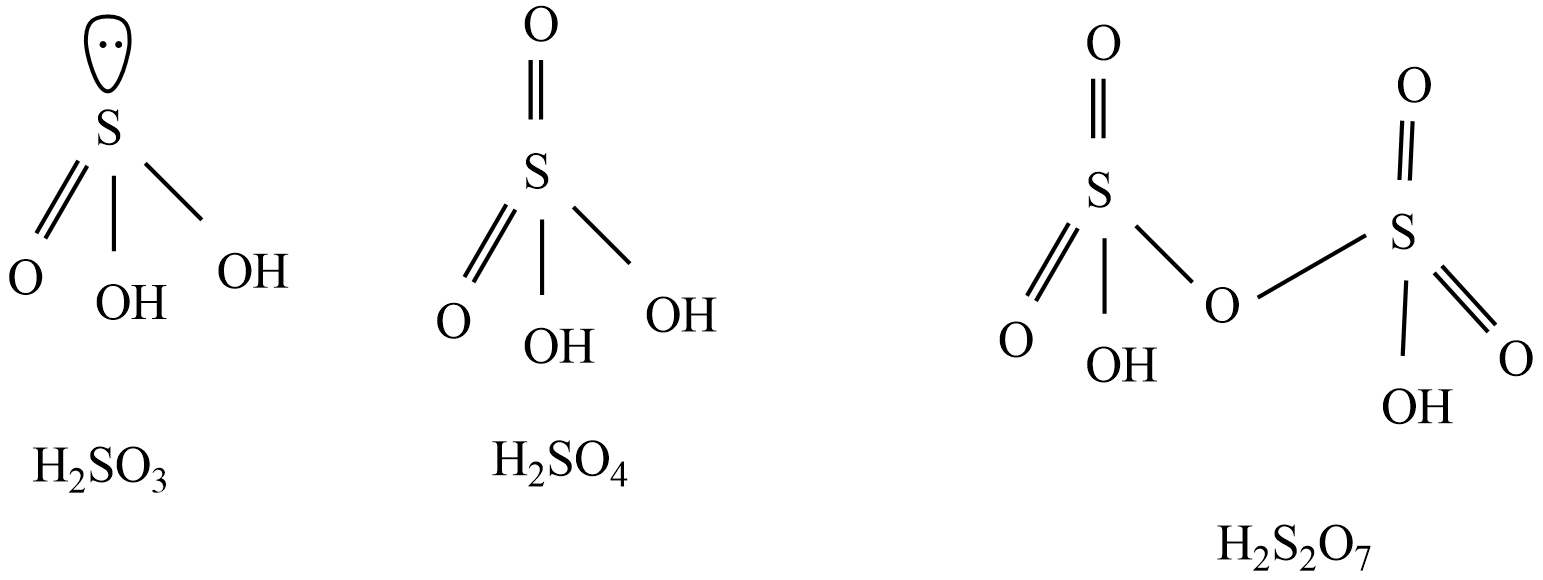
Form the above structures, H2SO3 ,H2SO4 , and H2S2O7 are diprotic acids, because two protons are attached to oxygen atoms in given oxoacids of sulfurs.
Observe the structures of H2CO3,H3BO3,H2CrO4
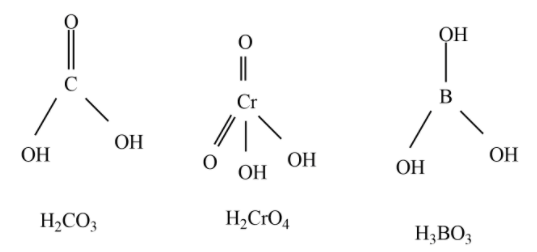
Out of the above three oxoacids, H2CO3,H2CrO4 are diprotic acids.
Hence, among the given oxoacids, six compounds are diprotic acids which are H2SO4,H3PO3,H2CO3,H2S2O7,H2CrO4,H2SO3 .
Note:
In oxoacids, polyprotic acids contain more than one proton attached to an oxygen atom. There will be a complex equilibrium in polyprotic acids due to the presence of multiple species in solution. Determination of the concentrations of different species at equilibrium can be very difficult due to the variety of possible ionic species in solutions for each acid in polyprotic acids.
