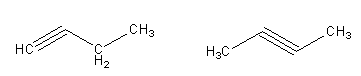Question
Question: The total number of cyclic isomers possible for hydrocarbon, \({{\text{C}}_{\text{4}}}{{\text{H}}_6}...
The total number of cyclic isomers possible for hydrocarbon, C4H6 is,
A. 9
B. 5
C. 11
D. 12
Solution
The molecules having the same molecular formula but a different arrangement of atoms are known as isomers. First, we will determine the degree of unsaturation, so we will know the number of unsaturated bonds. Then we will draw the possible geometries.
Complete solution:
First we will calculate the degree of unsaturation as follows:
D.U = C+1−2no. of monovalent−no. oftrivalent
On substituting 4 for C and 6 for no. of monovalent,
D.U = 4+1−26
D.U = 4+1−3
D.U = 2
So, the degree of unsaturation is2. So, we have the possibility of the presence of two double bonds or one triple bond in the structure. We also have the possibility of one ring and one double bond or two rings in the structures.
As we have to find the cyclic isomers only, we will find the structures having one ring with one double bond or two rings.
Structure with two rings:
The possible geometry of four carbon atoms with two ring is shown as follows:
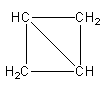
The above structure has two three-membered rings and C4H6 molecular formula.
Structure with one ring and one double bond:
We can draw the structure having one four-membered ring and one double bond as follows:
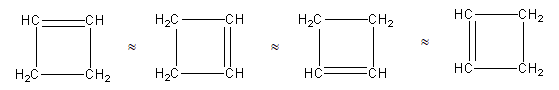
Above all four structures are the same as all four have the same chemical formula.
The highest possible ring size with four carbon is of a four-membered ring which we had drawn already, so we will try with three-membered cyclic structures.
The possible geometry of four carbon atoms with one ring and one double bond is shown as follows:
We can draw a three-membered cyclic structure with one double bond and one methyl group.
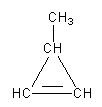
The second possibility is that we can draw the double bond outside of the ring as follows:
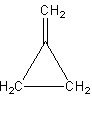
The third possibility is that we can shift the position in the ring as follows:
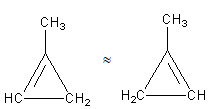
The above structures are the same as both have the same chemical formula.
So, the total number of cyclic isomers possible for hydrocarbon, C4H6 is, 5.
Therefore, the correct answer is (C).
Note: Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different group of atoms of a molecule. Here, C4H6is the molecular formula but the CH3CCHCH2 is the chemical formula. By the general formula of hydrocarbon, we will decide that the given molecular formula is representing the alkane, alkene, or alkyne. The general formula of the alkyne is, CnH2n - 2 .Where, n is the number of carbon atoms. So, C4H6 is alkyne. So, we can also draw the non-cyclic isomers having a triple bond.