Question
Question: The total number of basic groups in the following form of lysine is: 
A) 0
B) 1
C) 2
D) 3
E) 4
F) 5
G) 6
H) 7
I) 8
J) 9
Solution
Recall the definition of a base. According to the Bronsted-Lowry acid-base theory, a base is a substance that can accept hydrogen cations (H+). Examine the given structure of lysine to find the groups that can easily accept H+ ions and that will be the basic groups.
Complete step by step answer:
The given structure of lysine is as follows:
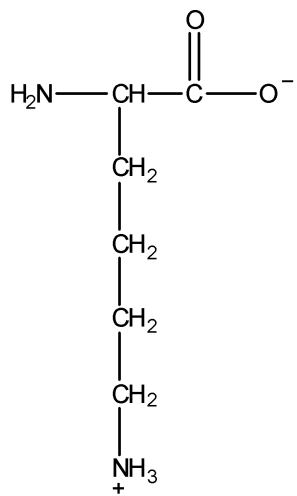
To find basic groups, first we should have knowledge of a base. There are many theories which define base but the general theory which is required to answer this question is Bronsted-Lowry acid-base theory. According to the Bronsted-Lowry acid-base theory, a base is a substance that can accept hydrogen cations (H+), also known as protons. So, the basic group in the structure of lysine would be the one which can accept H+ ions easily.
In the given structure of lysine, −COO group has a negative charge on one oxygen atom, and hence this oxygen atom can easily accept a hydrogen cation (H+) to become more stable OH. Thus, −COO group will try to accept H+ ion to become more stable −COOH. Hence, we can say that −COO group is a basic group in the given structure of lysine.
Now, there is a −NH2 group in the structure. We all know that nitrogen has a lone pair on it. −NH2 group is an incredibly strong base because it can accept a proton or H+ ion to become NH3 (ammonia) which is more stable. Hence, −NH2 group is another basic group.
Hence, there are two basic groups in the given structure of lysine.
So, the correct answer is “Option C”.
Note: Lysine (symbol: Lys) is an α-amino acid and is basic in nature. It is a basic amino acid because it has more number of basic groups than acidic groups. Also, lysine is one of the essential amino acids since the human body cannot synthesize it.
