Question
Question: The third line of the Balmer series in the emission spectrum of the hydrogen atom is due to the tran...
The third line of the Balmer series in the emission spectrum of the hydrogen atom is due to the transition from the?
a.) Forth bohr orbit to the first bohr orbit
b.) Fifth bohr orbit to the secondary bohr orbit
c.) Sixth bohr orbit to the third bohr orbit
d.) Seventh bohr orbit to the third bohr orbit
Solution
Hint: Balmer lines appear in numerous stellar objects due to the abundance of hydrogen in the universe. The longest wavelength in the balmer series is 656. The balmer series is atomic hydrogen. The wavelength of these lines are :1/λ=RH(1/4−1/n2), where λ is a wavelength, RH is Rydberg constant, and n is the original orbit level.
Complete answer:
In this given question the term emission spectrum means an electron which goes from high orbit to low orbit. As we know that in balmer lines this is fixed to (n=2) as you can see in the figure given below. According to the question the first option shows the lyman series not the balmer series. In the Lyman series we talk about the first orbit therefore, the wavelength of the first line of the Lyman series in hydrogen atom is ‘1216’. The second option (b) is correct because in that we are talking about the balmer series. Moreover, the option(c, d) we are talking about is the paschen series which means (n=3). Whereas the shortest wavelength of paschen series is 107m−1 and the longest wavelength of paschen series is 18752Ao 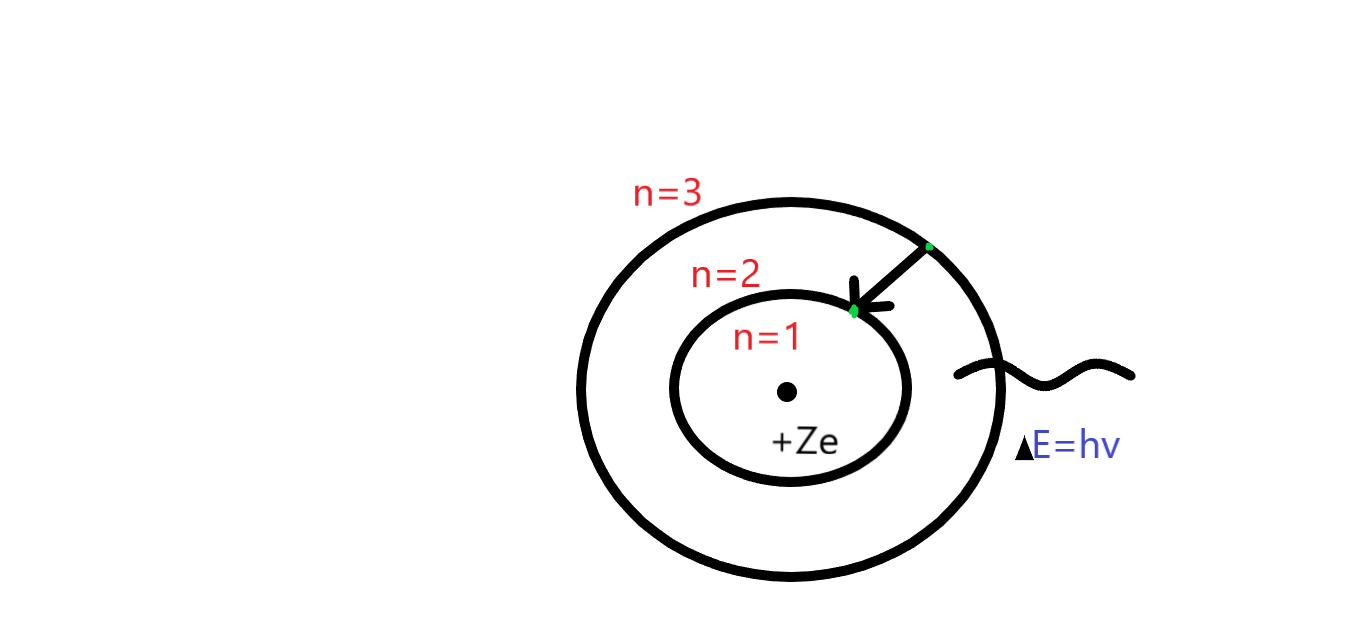
Note: In lyman series the main spectral lines are visible as ultra-violet, paschen, brackett and pfund all these series the main spectral lines are visible as infra-red. In Lyman series n=1, balmer series n=2, paschen series n=3, barckett series n=4, pfund series n=5.
