Question
Question: The tetrahedral shape is. A-\[s{p^2}\] B-\[s{p^3}\] C-\[ds{p^2}\] D- None of the above....
The tetrahedral shape is.
A-sp2
B-sp3
C-dsp2
D- None of the above.
Solution
We know that if a molecule has four bonds and no lone pairs around the central atom then the molecule has tetrahedral geometry and the bond angle is 109.5∘. Ammonium ion and the methane molecule are some of the examples of the tetrahedral molecule..
Complete step by step answer:
First, we discuss hybridization.
Hybridization means the mixing of two atomic orbitals of the same energy to give a new hybrid molecular orbital of the same energy. Based on the type of orbitals involved, hybridization is classified as sp,sp2,sp3,dsp2,dsp3 etc.
Let us discuss the shape and bond angle of given hybridization with examples.
A-sp2 Hybridization.
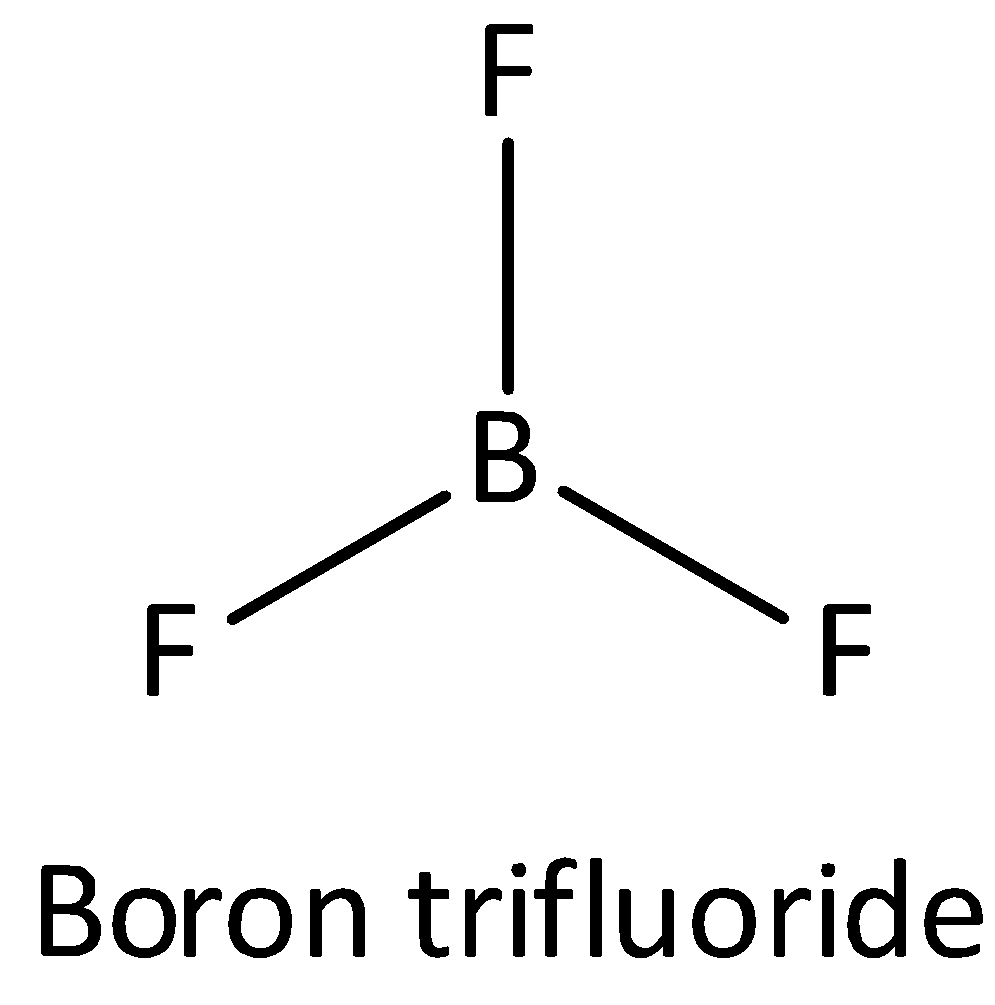
If one s and two p orbitals having the same energy mixed to form three equivalent hybrid orbitals then the newly formed orbital is known as sp2 orbital. An example of sp2 hybridized molecule is Boron trifluoride. The bond angle of sp2 orbital is 120∘. The shape of sp2 hybridized molecule is a trigonal planar. Thus option (A) is incorrect.
The structure of BF3 is,
B-sp3 Hybridization.
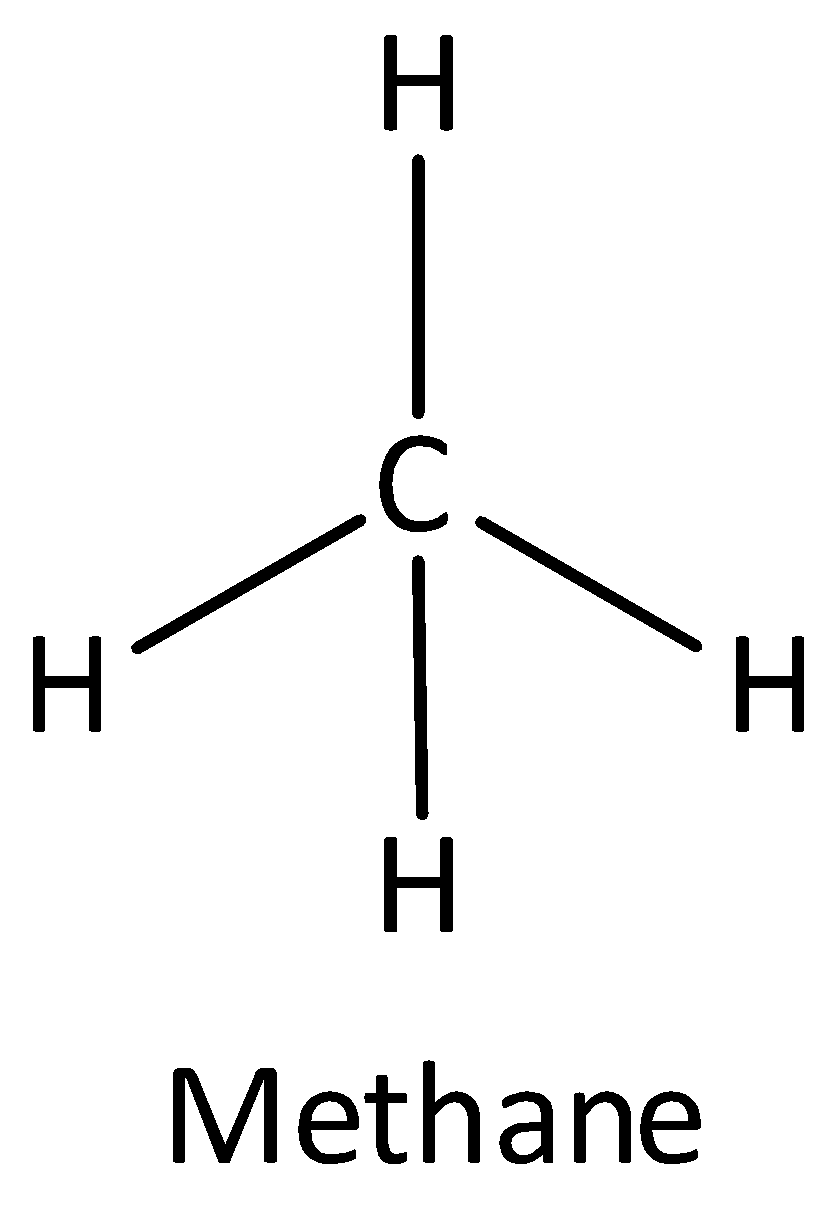
If one s and three p orbitals having the same energy mixed to form four equivalent hybrid orbitals then the newly formed orbital is known as sp3 orbital. An example of sp3 hybridized molecule is methane. The bond angle of sp3orbital is 109.5∘ . The shape of sp3 hybridized molecule is a tetrahedral. Thus option (B) is correct.
The structure of methane is,
C-dsp2 Hybridization.
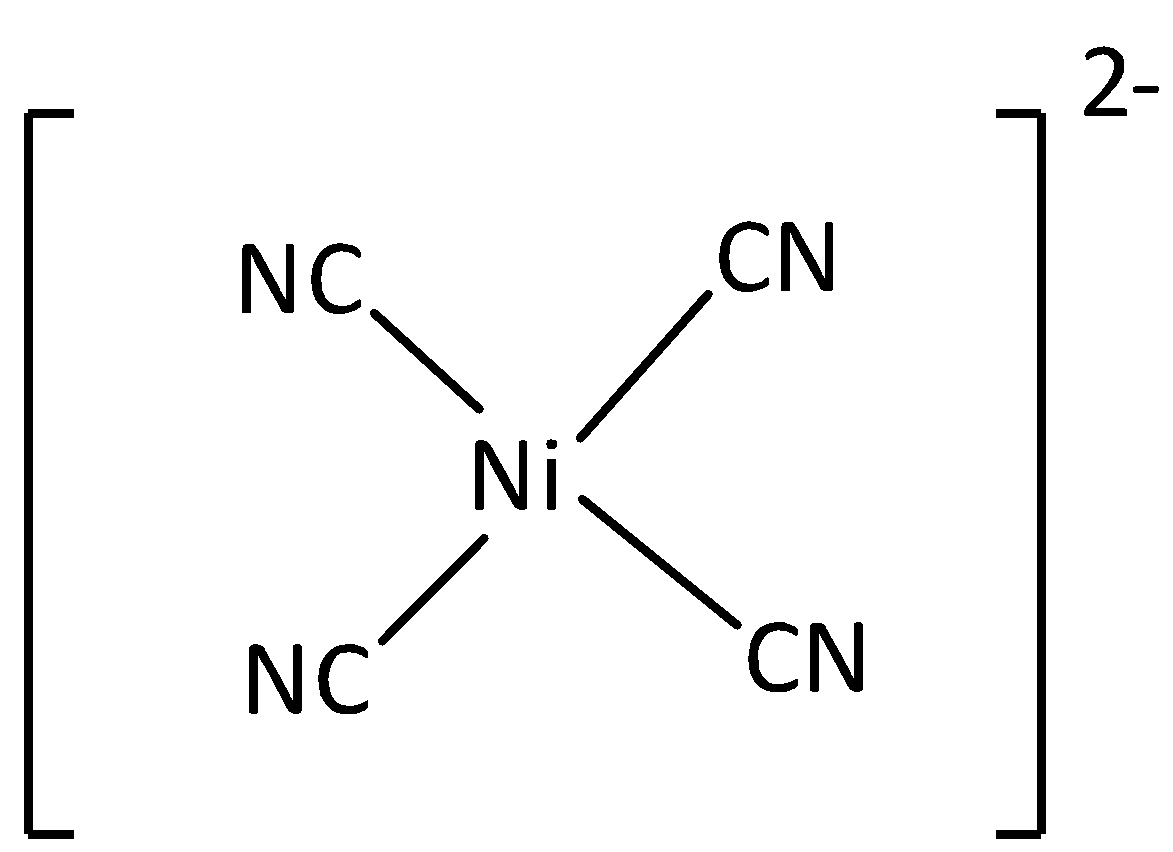
If one d , one s , and two p orbitals having the same energy mixed to form four equivalent hybrids orbital then the newly formed orbital is known as dsp2 orbital. An example of dsp2 hybridized molecule is nickel complex. The bond angle of dsp2orbital is 90∘. The shape of dsp2 hybridized molecule is a square planar. Thus option (C) is incorrect.
The structure of [Ni(CN)4]2− is,
Option B is the correct answer for this question.
Note:
We must know that the shape of a molecule can be identified by calculating the steric number. The total number of bonded and lone pairs of electrons in a molecule is called a steric number. It can be calculated using the formula,
Stericnumber=2Valenceelectronsofthecentralatom+numberofBondedatom
Let us calculate the steric number of methane. Using the formula,
Stericnumber=24+4=4
The steric number of methane is four. Thus it has four bonded atoms and no lone pair around the central atom. Thus it is sp3 hybridized tetrahedral molecule.
