Question
Question: The terminal C atom in butane is _______ hybridized. (A) \(ds{{p}^{2}}\) (B) \(sp\) (C) \(s{{...
The terminal C atom in butane is _______ hybridized.
(A) dsp2
(B) sp
(C) sp2
(D) sp3
Solution
There are 2 terminal carbons in the butane chain and both of them have the same hybridization. Both the terminal carbon atoms form 4 single bonds.
Complete step by step solution:
Butane is a member of the alkane group. It has 4 carbons. It has a straight-chain structure.
The formula of butane is C4H8
Since it is a member of the alkane group, all the bonds in butane will be a single bond.
So the structure of butane is given below:
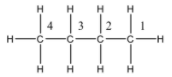
So, there are two terminal carbon atoms in the butane, carbon number one and four.
Both the carbon atoms are connected to three hydrogen atoms and one propyl group.
There are some steps which can help to calculate the hybridization of an atom:
First, look at the atom and count the number of atoms or molecules to which it is connected. If the atom has lone pairs, then it is also counted.
Add both numbers and:
If the number is 4 then it is sp3hybridized.
If the number is 3 then it is sp2hybridized
If the number is 2 then it is sphybridized
So, in both the terminal carbon atoms of butane the number is 4 (three hydrogen atoms and one propyl molecule).
Hence, the hybridization is sp3.
So, the correct answer is an option (d)- sp3.
Note: sp2hybridization mostly occurs when the carbon atom has a double bond and sphybridization occurs mostly when the carbon atom has a triple bond. dsp2hybridization is shown by heavier elements because they have vacant d-orbitals.
