Question
Question: The terminal and bridged \({\text{CO}}\) ligands in the compound \(\left[ {{\text{C}}{{\text{o}}_{\t...
The terminal and bridged CO ligands in the compound [Co2(CO)8] are respectively:
A. 0,2
B. 6,1
C. 5,2
D. 6,2
Solution
Calculate the total electrons for the complex. Place the bonds in such a way that the total electron count is satisfied.
The metal atom Co contributes nine electrons each.
The CO ligand at terminal position donates two electrons to each metal and one electron to each metal when bridged.
Complete step by step answer:
Step 1:
Calculate the total electrons for the complex [Co2(CO)8].
The metal Co contributes nine electrons each while the ligand CO contributes two electrons each. Thus,
Total electron count for [Co2(CO)8] =(2×Electrons of Co)+(8×Electrons of CO)
=(2×9)+(8×2)
=18+16
Total electron count for [Co2(CO)8] =34
Step 2:
Calculate the number of metal – metal bonds per metal:
Number of metal - metal bonds per metal =2(Number of metal atoms×18)−Total electron count
The complex [Co2(CO)8] has 2 Cometal atoms. Thus, substitute 2 for the number of metal atoms and 34 for the total electron count. Thus,
Number of metal - metal bonds per metal =2(2×18)−34
=236−34
=22
Number of metal - metal bonds per metal =1
Step 3:
Draw the structure for the complex [Co2(CO)8]:
There is one metal – metal bond i.e. there is one bond between Co−Co.
Each Co atom contributes nine electrons.
The CO ligand at terminal position donates two electrons to each metal and one electron to each metal when bridged. Thus, the structure for the complex is,
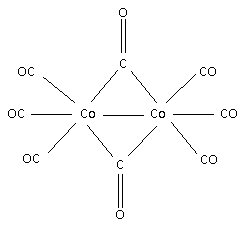
Step 4:
Calculate the number of terminal and bridged CO ligands.
From the structure,
There are 6 terminal CO ligands and 2 bridged CO ligands.
Thus, the terminal and bridged CO ligands in the compound [Co2(CO)8] are 6 and 2 respectively.
Thus, the correct option is option (D).
Note: The metal atom Co contributes nine electrons each. The CO ligand at terminal position donates two electrons to each metal and one electron to each metal when bridged. Placing the bonds accurately to satisfy the total electron count gives the exact number of terminal and bridged ligands.
