Question
Question: The temperature inside and outside of a refrigerator are \(275 \) K and \(300\) K, respectively. Ass...
The temperature inside and outside of a refrigerator are 275 K and 300 K, respectively. Assuming that the refrigerator cycle is ideal reversible, for every joule of work done the heat delivered to the surroundings is nearly.
a) 10J
b) 11J
c) 14J
d) 12J
Solution
The working substance absorbs some amount of heat from the sink which is at the lower temperature, then W is the work which is done on the working substance and then some amount of heat rejected by the working substance to the surroundings. This is the basic principle of refrigerators.
Complete step by step answer:
Given inside temperature (sink temperature), T2=275K
Outside temperature (source temperature), T1=300K
Work done = 1J
R is the refrigerator.
Assume: Refrigerator is ideal and reversible. So, we can write:
Q2Q1=T2T1
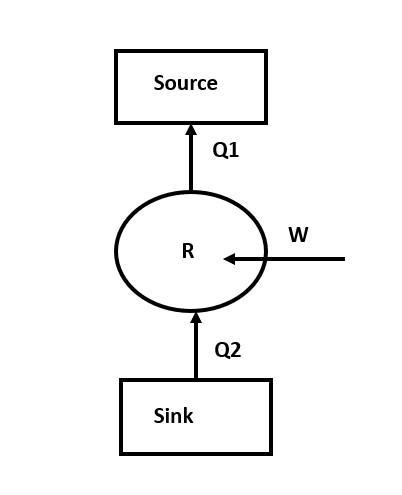
We have to find the heat lost to the surroundings, Q1.
Coefficient of performance -
B=WQ2
Now, we will write B in terms of the temperatures.
B=T1−T2T2
B=300−275275=11
By using, formula of coefficient of performance, we can evaluate: -
B=WQ2
Now, we will put the value of W.
10=1Q2
Q2=10j
By using the value of work, we can calculate Q1.
W=Q1−Q2
Q1=W+Q2
Q1=1+10=11J
So, the heat delivered to the surroundings = 11J.
So, the correct answer is “Option b”.
Note: The heat flow follows the first law of thermodynamics. Work has to be done, otherwise heat will not flow from low temperature to high temperature and violates the second law of thermodynamics. In the refrigerator, heat is rejected to surroundings and entropy is increased.
