Question
Question: The table below shows the specific heats of several metals. The temperature of a 15-g sample of an u...
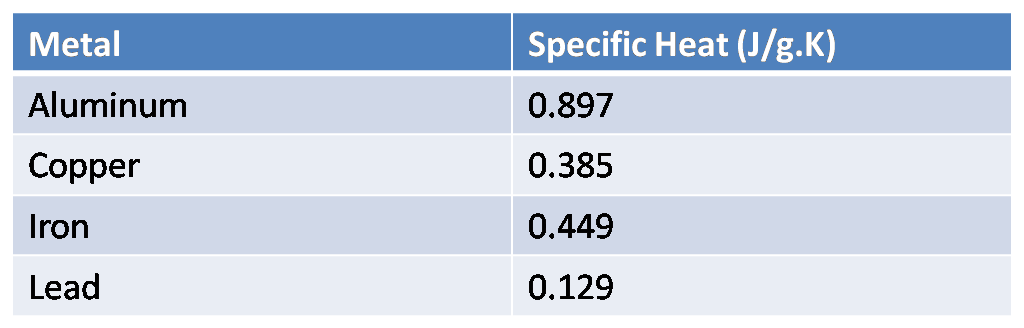
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from 20∘C to 30∘C when it absorbs 67.5 J of heat. Which of the metals in the table below could be the unknown metal?
Solution
Specific heat tells us about heat needed for the rise in temperature by one degree Celsius by one gram of any element. Specific heat and heat are two different entities related to each other.
Formula used:
Formula for heat energy, Q=mcΔT
Complete answer:
We are given the specific heats of various metals in the table. Given the data that,
Heat absorbed = 67.5 J
Mass of the sample = 15 g
Temperatures = 20∘Cto30∘C so, ΔT= 30 – 20 = 10∘C
We have to find from this given data, the metal from the table that possesses the specific heat as obtained by this given information.
As formula for heat absorbed is Q=mcΔT
Keeping the given data in this formula, we get:
67.5 = 10gc×10∘C
c = 15g×10∘C67.5J
c = 0.45 J/ g.K
This value of specific heat is around the specific heat of iron from the table.
Hence, iron metal from the table could be the unknown metal.
Note:
The temperature difference, ΔT, taken out in Celsius will be the same as taken out for Kelvin. The specific heat in the answer does not match any quantity in the table, so we use that quantity of specific heat which is most close to the calculated value, therefore, iron is the answer.
