Question
Question: The system shown in figure consists of three springs and two rods as shown. If the temperature of th...
The system shown in figure consists of three springs and two rods as shown. If the temperature of the rods is increased by ΔT , calculate the energy stored in each of the springs. The springs are initially relaxed. There is no friction. Take the coefficient of linear expansion of the material of rods to be equal to α -
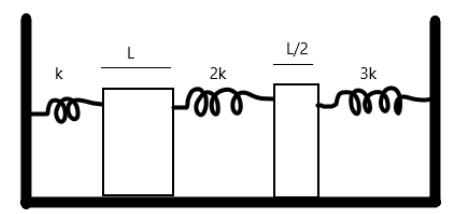
Solution
Hint : The extension of the length of the rod will cause an extension in the string. The rod will extend equally in both left and right directions. Observe that the middle spring will be doubly compressed due to the extension of the two rods.
Formula used: In this solution we will be using the following formulae;
E=21ke2 where E is the energy stored in a spring, k is the spring constant of the spring and e is the extension or compression of the spring from equilibrium position.
α=LΔTΔL where α is the coefficient of linear expansion of a material, ΔL is the increase in length of the body, L is the initial length and ΔT is the change in temperature of the substance.
Complete step by step answer
To calculate the energy stored in each of the springs, we note that the extension in length due to the change in temperature of the substance causes the springs to compress. Hence, we must calculate the extensions. The coefficient of linear expansion can be given by
α=LΔTΔL where ΔL is the increase in length of the body, L is the initial length and ΔT is the change in temperature of the substance.
So for the rod of length L , we have a change in length of
ΔL1=αLΔT
And hence similarly for the rod of length 2L
ΔL2=α2LΔT
Now, the length increases equally in both directions. Hence the compression in the first spring is 2ΔL1
The energy in a spring can be given by E=21ke2 where k is the spring constant of the spring and e is the extension or compression of the spring from equilibrium position.
Hence, the energy of first spring is
E=21k(2ΔL)2
Then, by inserting the expression for ΔL , we have
E=21k(2αLΔT)2=81kα2L2ΔT2
For the second spring, it is compressed by 2ΔL1 from the left, and by 2ΔL2 from the right, hence the total extension is 2ΔL1+2ΔL2
Then,
E2=212k(2ΔL1+2ΔL2)2
Inserting known expressions, we get
E2=212k2αLΔT+2α2LΔT2=212k(43αLΔT)2
By simplification
E2=169kα2L2ΔT2
For the third spring, the extension is 2ΔL2
Hence,
E3=213k2α2LΔT2
Hence, by simplification, we have
E3=213k(4αLΔT)2
⇒E3=213k(4αLΔT)2=323kα2L2ΔT2 .
Note
For clarity, note that the substance is assumed to have increased equally in both directions. This happens when the temperature is evenly distributed along the line parallel to the length. In such a case where it isn’t the assumption in invalid.
