Question
Question: The sum of the number of lone pairs of electrons on each central atom in the following species is ...
The sum of the number of lone pairs of electrons on each central atom in the following species is
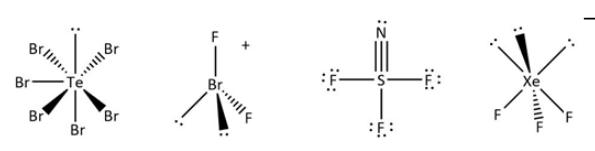
{{\rm{[TeB}}{{\rm{r}}_{\rm{6}}}]^{2 - }},\;{[{\rm{Br}}{{\rm{F}}_2}]^ + },\;{\rm{SN}}{{\rm{F}}_3},{\rm{ and\;[Xe}}{{\rm{F}}_{\rm{3}}}{]^ - }\\\ \left[ {{\rm{Atomic numbers}}:\;{\rm{N}} = 7,\;{\rm{F}} = 9,\;{\rm{S}} = 16,\;{\rm{Br}} = 35,\;{\rm{Te}} = 52,\;{\rm{Xe}} = 54} \right] \end{array}$$Solution
As per VSEPR theory this can be solved. We know that VSEPR theory explains the geometry of the molecule. According to this theory, the geometry of the molecule is dependent on the number of bonding and number of non-bonding electron pairs in the central atom.
It is also known to us that the strength of the repulsion between a lone pair and a bond pair of electrons lies in between the repulsion between two lone pairs and between two bond pairs. The order of repulsion between electron pairs as follows: Lone Pair- lone pair > Lone Pair- bond- pair > Bond Pair- bond pair. The electron pairs around the central atom repel each other and move so far apart from each other that there are no greater repulsions between them. This results in the molecule having minimum energy and maximum stability.
Formula used:(a) To find total pairs the formula will be: 21 (number of valence electrons of central metal atom + number of atoms attached to central metal – overall charge).
(b) Lone pairs (Ip) = Total pairs (T) - Bond pairs (B).
Complete step-by-step answer: The atomic number of Te atom is 52 with electronic configuration [Kr]4d105s25p4, it has six valence electrons hence it will form six bond with bromine. By using this formula (a) and formula (b) we get a number of lone pairs of electrons.
[TeBr6]2−,T=21(6+6+2)=7,B=6.
Te atom in [TeBr6]2− has 1 lone pair of electrons. Same way we can do it for other molecules: the number of valence electrons for bromine are seven, and two fluorines are attached to it with one positive charge.
{[{\rm{Br}}{{\rm{F}}_2}]^ + },{\rm{T}} = \frac{1}{2} = \left( {7 + 2 - 1} \right) = 4,{\rm{B}} = 2$$$${\rm{Br}} atom in[BrF2]+has 2 lone pairs of electrons.
Xenon has eight electrons in the valence shell. Three fluorines are attached with negative one charge. [XeF3]−,T=21(8+3+1)=6,B=3.Xe atom in[XeF3]− has 3 lone pairs of electrons.
Satom in SNF3has 0 lone pair of electrons. Can be concluded from the structure. Because nitrogen and fluorine have different numbers of bond formation with central metal ions.