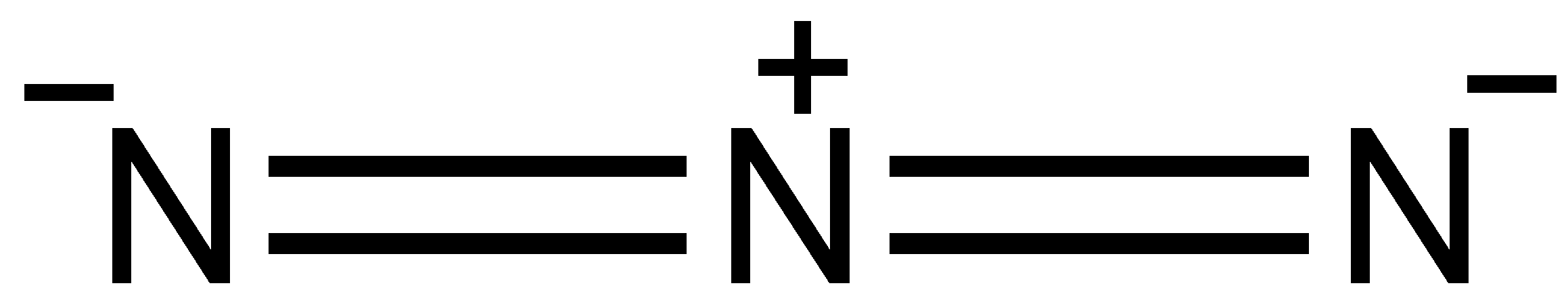Question
Question: The structures of \({O_3}\) and \(N_3^ - \) are: A) linear and bent, respectively B) both linear...
The structures of O3 and N3− are:
A) linear and bent, respectively
B) both linear
C) both bent
D) bent and linear, respectively
Solution
Recall the lewis representation of molecules. The lewis dot structure gives a picture of the bonding in molecules and ions in terms of the shared bonding pairs and follows the octet rule. Draw the lewis dot structures of O3 and N3− to get the answer.
Complete step by step answer:
Let us proceed in steps to draw the lewis structures of the molecule O3:
Step 1. Count the number of valence electrons of oxygen atoms. The outer valence shell electronic configuration of O is 2s22p4. Therefore, valence electrons of three oxygen molecules are 6 + 6 + 6 = 18.
Step 2. Draw the skeletal structure of O3 as: O O O
Step 3. Draw a single bond (one shared electron pair) between the two oxygen atoms completing the octet on each oxygen atom. However, we have to draw a double bond between two oxygen atoms. The remaining electrons after completing the octet on each atom constitute lone pairs.
We get the structure as:
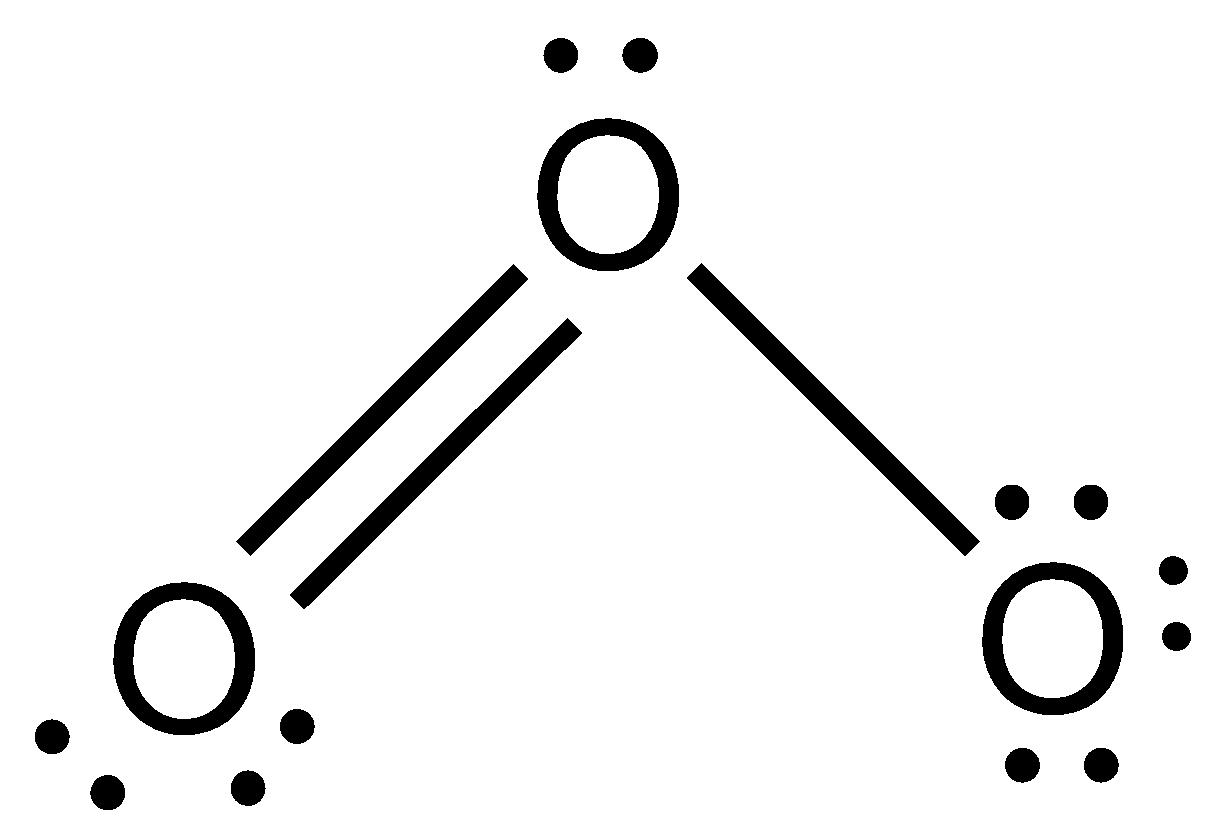
Thus, we get the structure of O3 as bent.
Now, lewis structures of the molecule N3−:
Step 1. Count the number of valence electrons of nitrogen atoms. The outer valence shell electronic configuration of N is 2s22p3. Therefore, valence electrons of three nitrogen atoms are 5+5+5 = 15.
Step 2. Draw the skeletal structure of N3− as: N N N
Step 3. Draw a single bond (one shared electron pair) between the two nitrogen atoms completing the octet on each nitrogen atom. However, we have to draw a double bond between two nitrogen atoms to complete the octets. The remaining electrons after completing the octet on each atom constitute lone pairs. There is a negative charge too on the molecule which means we need to add one extra electron in the structure. So, there are a total of 16 electrons in the N3− molecule. We can show negative charge on the whole sphere in the structure of molecules.
We get the structure as:
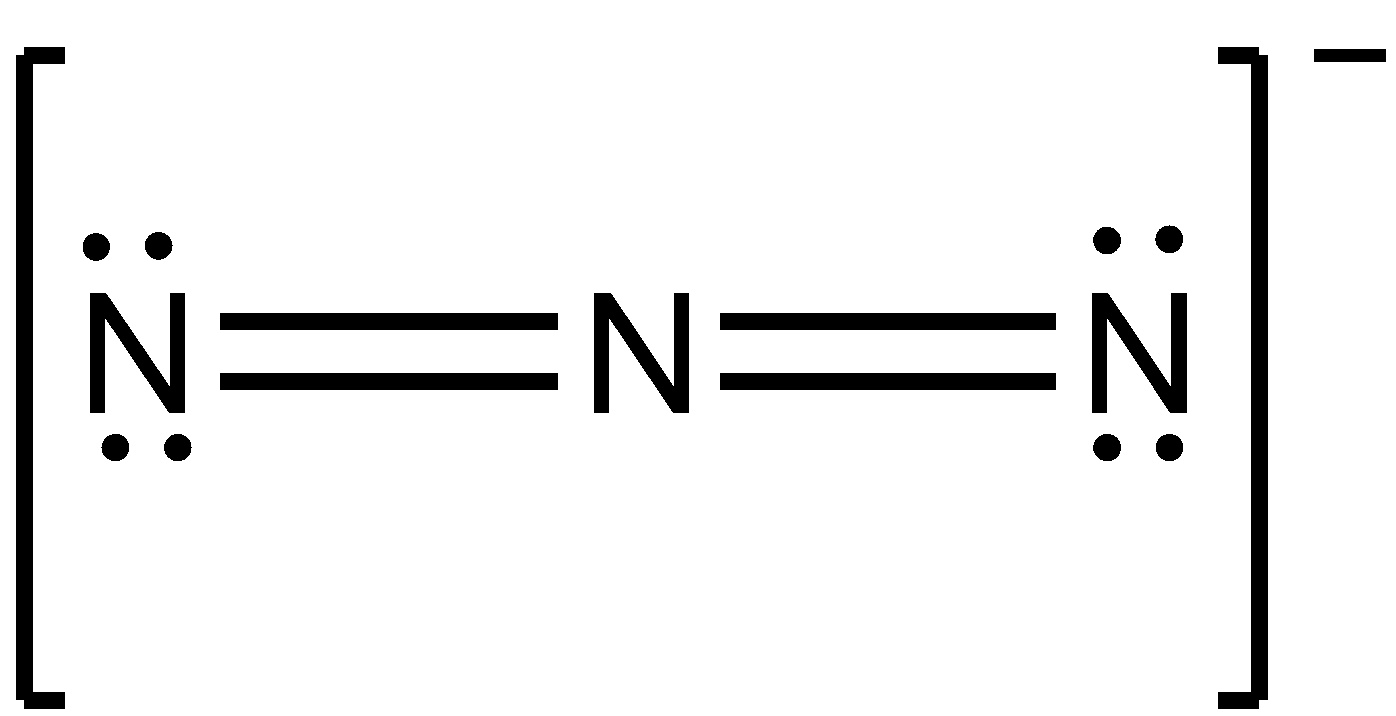
Thus, the structure of N3− molecule is linear in shape.
Hence, the structures of O3 and N3− are bent and linear respectively.
So, the correct answer is “Option D”.
Note: O3 is known as ozone and N3− is known as azide ion. Ozone molecules show two resonance structures. Azide ion can also show various resonance structures, but an important one is as shown below: