Question
Question: The structure of toluene is? A. 
B. 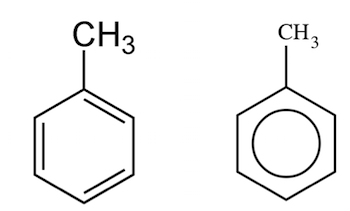
C. 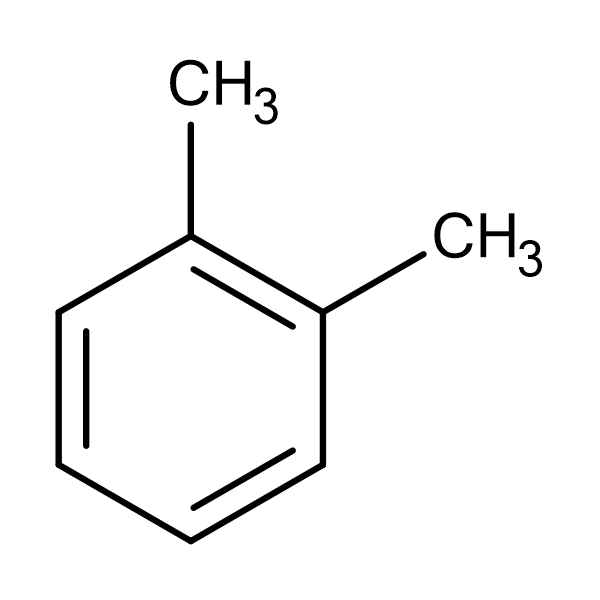
D. 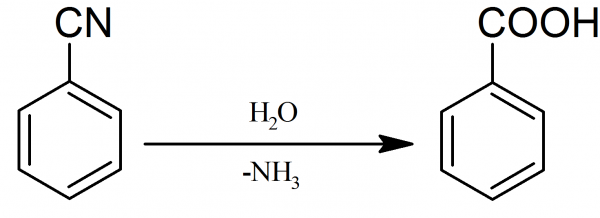
Solution
Hint: Toluene is a mono-substituted benzene derivative and it is more reactive than benzene toward electrophiles. We can use properties to identify the correct structure.
Complete step by step answer:
Toluene is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners.
It is a mono-substituted benzene derivative, consisting of a methyl group attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene.
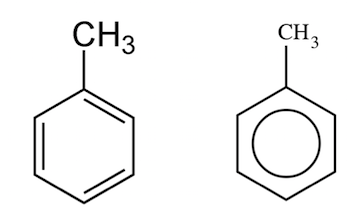
Which is given in option B .
We can also identify structures provided in other options too.
The structure in option A, is of ethylbenzene. It is also a mono-substituted benzene derivative.
The structure in option C, is of 1,2-dimethylbenzene. It is a di-substituted benzene derivative.
The structure in option D, is of benzoic acid. It is also a mono-substituted benzene derivative.
Thus, the correct option is B.
Additional information: Toluene is used as the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue.
Toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm.
Note: Toluene reacts as a normal aromatic hydrocarbon in electrophilic aromatic substitution. Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles.
