Question
Question: The structure of the product S is: \[P\xrightarrow[\begin{smallmatrix} 2.H{}^{+},\text{ }{{H}_...
The structure of the product S is:
2.H{}^{+},\text{ }{{H}_{2}}O \\\ 3.{{H}_{2}}S{{O}_{4}},\Delta \end{smallmatrix}]{1.MeMgBr}Q\xrightarrow[2.Zn,{{H}_{2}}O]{1.{{O}_{3}}}R\xrightarrow[2.\text{ }\Delta ]{1.O{{H}^{-}}}S$$ A.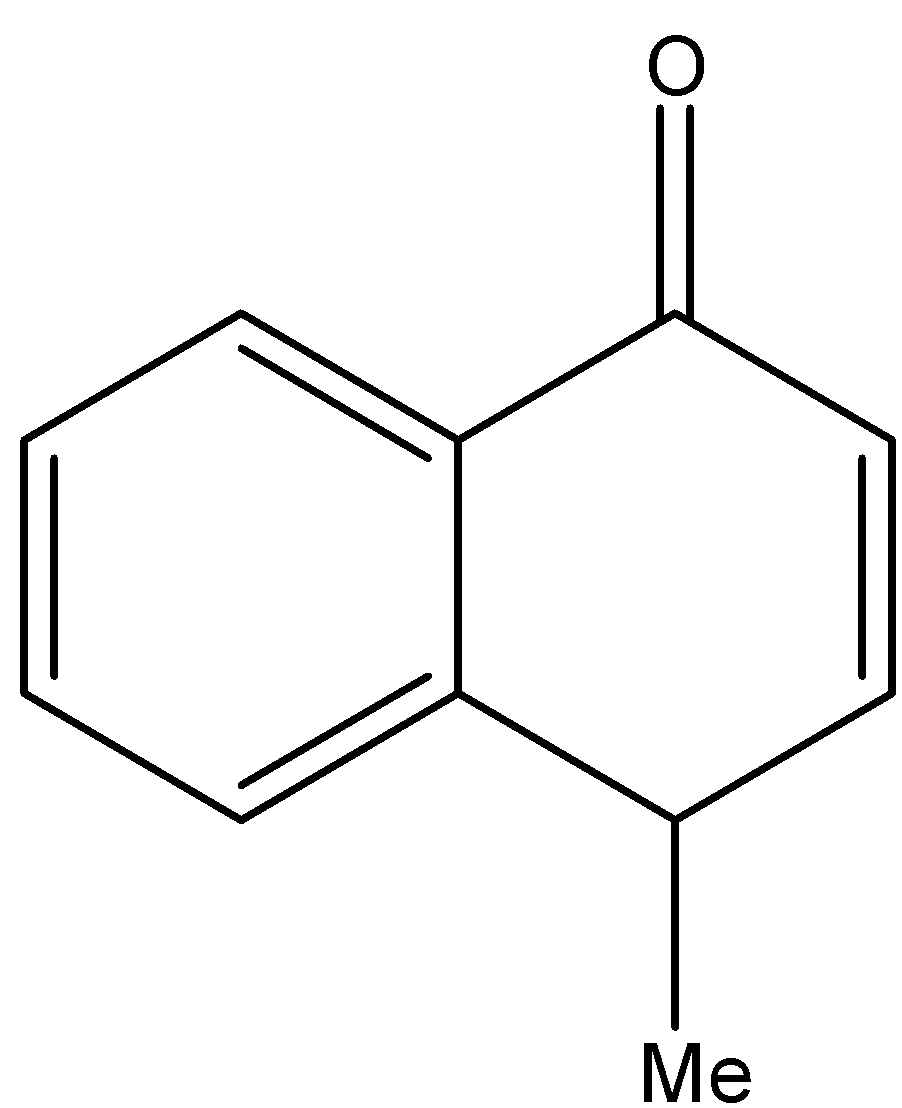 B. 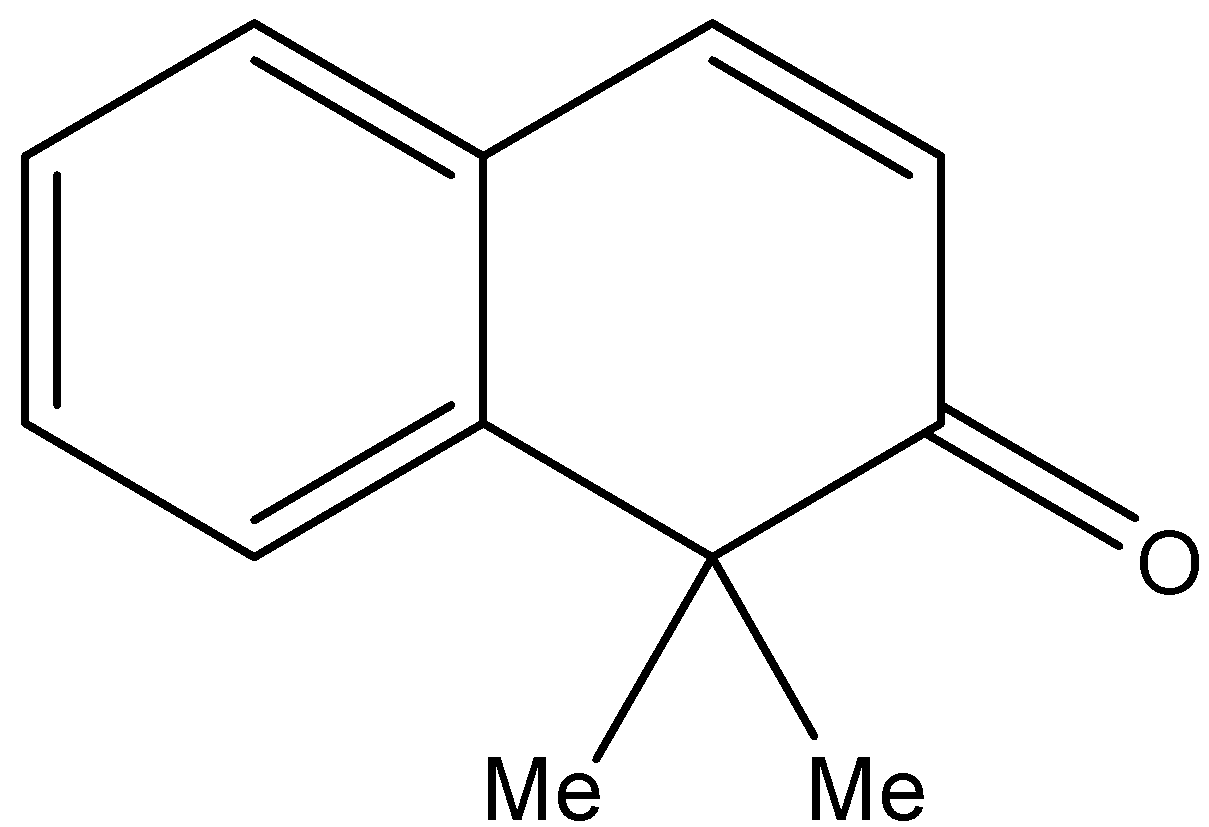 C. 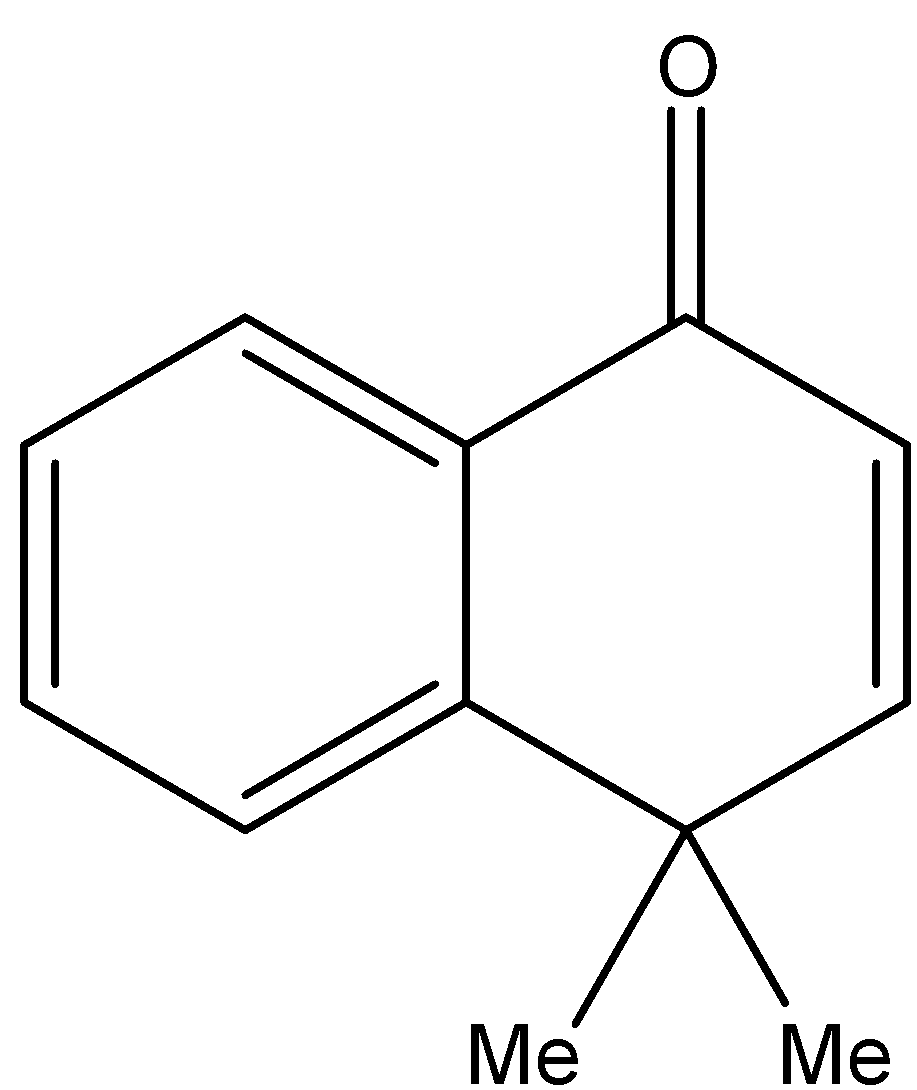 D. 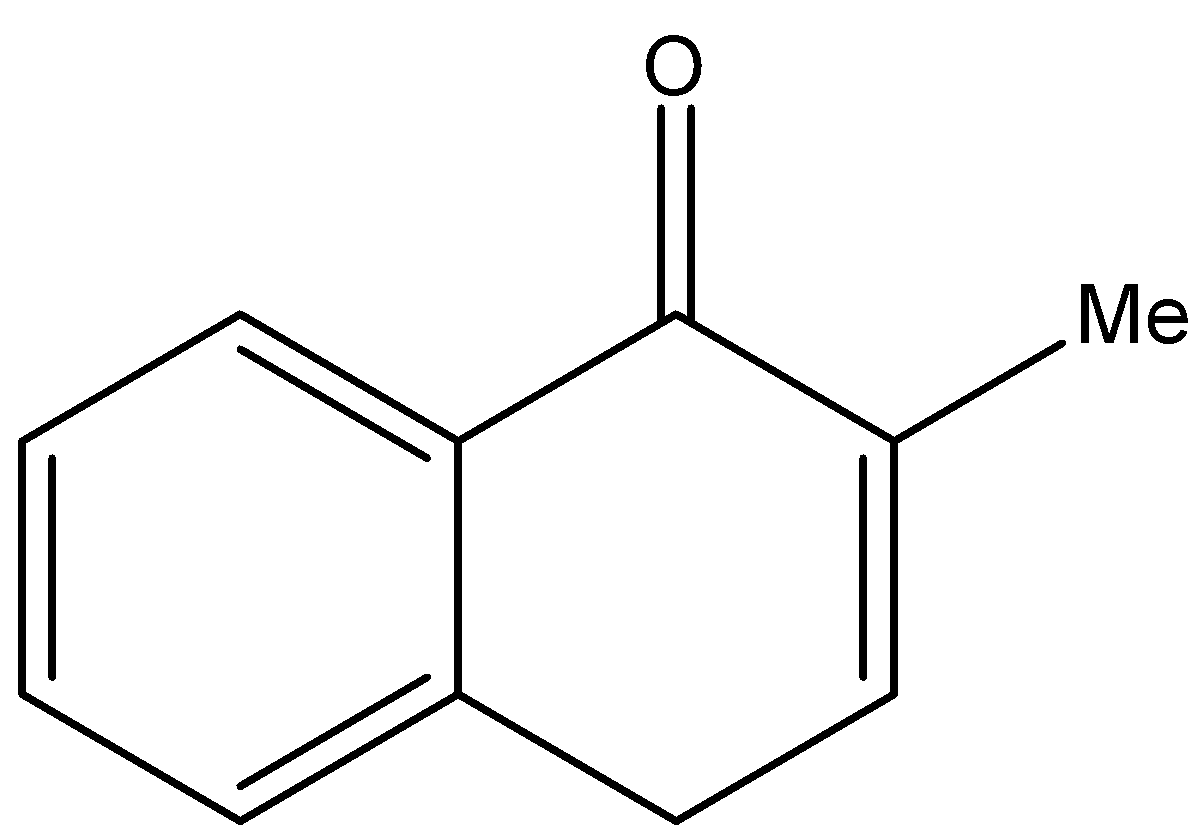Solution
Methyl ketones respond to iodoform test and form respective carboxylic acid and iodoform as the products. It is a test used to find the presence of methyl ketones in the given compounds. Generally methyl ketones react with Grignard reagent and form respective alcohols as the products.
Complete answer:
- In the question it is given that we have to find the structure of the Compound S.
- The compound P is a methyl ketone that reacts with Grignard reagent and forms a respective alcohol as an intermediate compound and it undergoes dehydration reaction in presence of an acid and forms product Q.
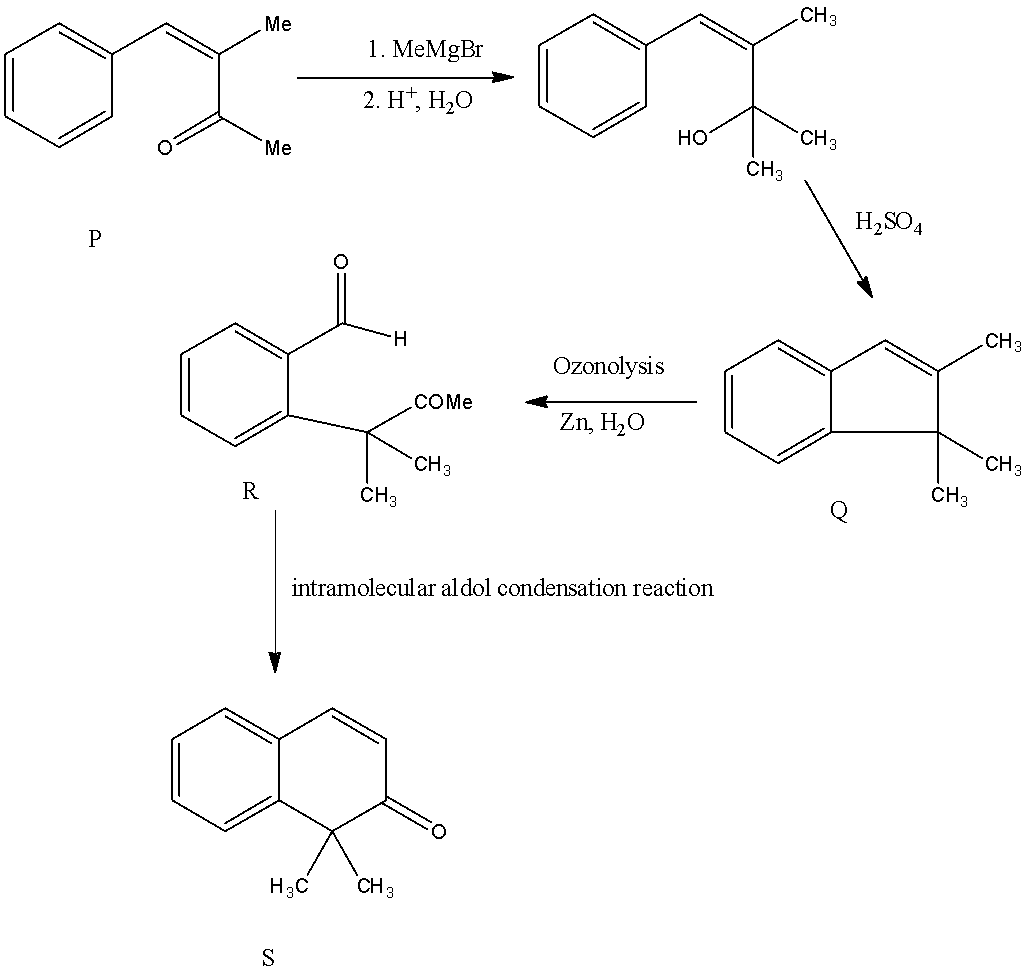
- Due to the presence of unsaturation (double bond) in the compound Q, it undergoes ozonolysis and forms a compound R containing an aldehyde and a ketone in its structure.
- Later the compound R undergoes an intramolecular aldol condensation reaction and forms a cyclic ketone compound S as the product.
By checking the given options the compound S is identified as option B.
Note:
If the compound R formed as an intermediate product undergoes intramolecular Cannizaro reaction then it forms a compound containing a carboxylic group and an alcohol in the structure of the compound S. If the compound undergoes an intramolecular aldol condensation then only a cyclic compound is going to form as the product. The compound formed through Cannizaro reaction contains a carboxylic functional group and an alcohol functional group in its structure.
