Question
Question: The structure of the compound with molecular formula \({C_6}{H_{12}}\) that has only primary and sec...
The structure of the compound with molecular formula C6H12 that has only primary and secondary H atoms is:
a.) 1-Methyl cyclo-pentane
b.) 1,1-Dimethyl cyclobutane
c.) Cyclohexane
d.) 2-Methyl pent-2-ene
Solution
Hint: In order to solve the given problem, first we will understand the concept behind the existence of different structures of organic compounds with the same chemical formula. Further we will understand the meaning of primary and secondary atoms in a compound. And finally on the basis of structure of the organic compounds given in the options we will find the correct answer.
Complete step by step solution: As we know that there may exist different organic compounds with difference in the arrangements of elements and difference in their structure but may have the same chemical formula. This concept is commonly known as isomerism.
In order to understand primary and secondary hydrogen atoms, let us first understand the basic concept of primary and secondary carbon atoms.
The carbon atom in any chemical compound directly attached to only one carbon atom is known as primary carbon or 10 carbon. And the carbon atom in any chemical compound which is directly attached to two other carbon atoms is known as secondary carbon or 20 carbon.
Any hydrogen atom attached to primary carbon in a compound is known as primary hydrogen atom. And those hydrogen atoms attached to secondary carbon atoms in a chemical compound is known as secondary hydrogen.
Now in order to solve the problem let us study the structure of the given compound. We will see the detailed structure of the correct answer with the help of figure and for other options we will analyse the chemical compound.
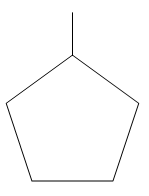
__
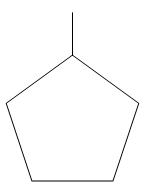
__ As we can analyse in the figure given above.
In option A: 1-Methyl cyclo-pentane there is 1 primary carbon, 4 secondary carbons and one tertiary carbon. There is also the presence of one hydrogen atom with the tertiary carbon. So option A is incorrect,__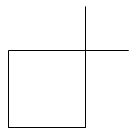
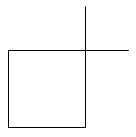
As we can analyse in the figure given above.
In option B: 1, 1-Dimethyl cyclobutane there is the presence of two primary carbons, three secondary carbons and one tertiary carbon. But there are no hydrogen atoms attached to tertiary carbon atoms. So the hydrogen atoms are attached only with primary and secondary carbon atoms.
Hence, the structure of the compound with molecular formula C6H12 that has only primary and secondary H atoms is 1,1-Dimethyl cyclobutane
So, the correct answer is option B.
Note: In order to solve such types of problems students must be aware of the concept of isomerism and also should know the method of drawing figures for different chemical compounds. On the basis of diagrams of the chemical compound, the analysis of such types of problems becomes easier.
