Question
Question: The structure of \(S{{O}_{3}}\) molecule in the gaseous phase contains: (A) Only \(\sigma \)- bond...
The structure of SO3 molecule in the gaseous phase contains:
(A) Only σ- bond between sulphur and oxygen
(B) σ bonds and a (pπ-pπ) bonds between sulphur and oxygen
(C) σbonds and a dπ- pπ) bonds between sulphur and oxygen
(D) σ bonds and a (pπ-pπ) bonds and a ( dπ- pπ) bonds between sulphur and oxygen
Solution
To answer this question we should be aware when σbonds, (pπ-pπ) bonds and ( dπ- pπ) bonds are formed. Valence electrons are the electrons that are present in the outermost shell of an atom upon this the covalency of the atom depends.
Complete answer:
Sulphur has six electrons in its outermost shell that is its covalency is six. Covalency of an atom is the number bonds an atom can make. In solid state the SO3molecule exists in a cluster that is the number ofSO3 molecules bonded together to form a solid structure. In the gas phase the SO3 molecule exists as a single molecule which is also said to exist in the free State.
σ- bond is formed by overlapping of atomic orbitals or hybrid orbitals in their axis.
For example: overlap of s-s, s-p,sp2-sp2 overlap along their axis.
π- bond is formed by the side to side overlap of molecular orbital or atomic orbital along a plane perpendicular to the plane. If the donating orbital is p and the orbital to which the electron pair is donated is p then it is known as Pπ-pπ.
Similarly, if the donating orbital is d and the orbital to which the electron pair is donated is p then it is known as dπ- pπ
Firstly, let's draw the Lewis structure ofSO3:
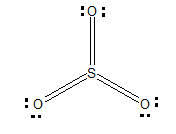
There are three delocalised π bonds, out of which one of them is Pπ-pπand other two are dπ- pπ. The sulphur is dπ−pπhybridized.
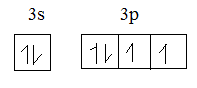
The electronic configuration of sulphur is 1s22s22p63s23p4.
Sulphur in ground state:
In excited state:
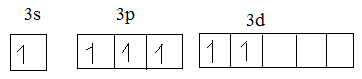
Oxygen has 6 electrons. So, the valence electrons of oxygen make bond with unpaired electrons.
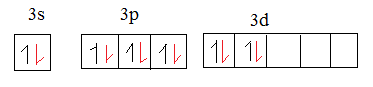
The 6 valence electrons in oxygen are of 2p orbital.
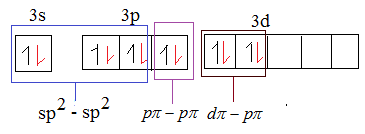
The red coloured electrons in the above mentioned diagram are the electrons of oxygen. The sp2-sp2 overlap forms σ bond as sulphur in SO3 is sp2 hybridized.
From the above diagram it is evident that option D is the correct answer.
Note: Knowing the valance electrons of a particular element may help us to figure out their structure. Always whenever, a double bond is formed. Here one of the bonds will be σ bond and the other will be π bond. Now, the π bond can be pπ-pπbond, dπ- pπbond or dπ-dπbond.
