Question
Question: The structure of \[PC{l_5}\] is a.) Trigonal bipyramidal b.) Octahedral c.) Pentagonal bipyram...
The structure of PCl5 is
a.) Trigonal bipyramidal
b.) Octahedral
c.) Pentagonal bipyramidal
d.) Square pyramidal
Solution
For finding out the hybridization of the given molecule we can use the hybridization formula, and by referring to the arrangement of spdf orbitals we can conclude the structure of the given molecular formula.
Complete step by step answer:
First consider which is the central atom, in this question our central atom is Phosphorous. Now, since the central atom has been found we will consider the hybridization formula.
- The general formula of hybridization is is given as
H=(V+X+C+A)/2
Where,
V= The number of valence electrons of the central atom.
X= The number of monovalent atoms attached to the central atom.
C=Total positive charge on the molecule.
A= Total negative charge on the molecule.
- Now, for PCl5 since we know that central atom is phosphorous and there are 5 chlorine atom surrounding it, and the number of valence electron of phosphorus is 5, then writing all the values;
Total number of valence electrons of Phosphorus; V=5
The number of fluorine attached to iodine; X=5
Total positive charge on the molecule; C=+0
Total negative charge on the molecule; A=−0
By putting all the above given values in the hybridization formula we get,
H=(5+5+0−0)/2
H=10/2,
H=5,
Now referring to the table given below:
| VALUES OF H | HYBRIDIZATION | STRUCTURE |
|---|---|---|
| 2 | sp | Linear |
| 3 | sp2 | Trigonal planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
By considering the above table we get to know that since our H is 5 the hybridization of PCl5 is trigonal bipyramidal.
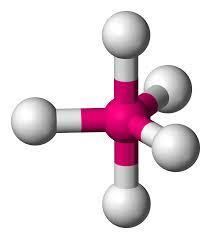
The correct answer is option “A” .
Note: In questions related to structures always remember to apply hybridization formula, and refer to the table given above, it is applicable to all compounds which have hybridization seven or less than it.
