Question
Question: The structure of \(P{F_5}\) molecule is. A) Square planar. B) Tetrahedral. C) Trigonal bipyram...
The structure of PF5 molecule is.
A) Square planar.
B) Tetrahedral.
C) Trigonal bipyramidal.
D) Pentagonal Bipyramidal.
Solution
We can find the geometry of a molecule by finding the steric number of a molecule. The steric number of molecule is calculated using the formula,
Stericnumber=2valenceelectronofcentralatom+no.of.bondedatom
Complete step by step answer:
Let us see what hybridization is.
Hybridization:
Hybridization is the idea that atomic orbitals combine to form new hybridized orbitals which in turn, influences molecular geometry and bonding properties.
We know that the electrons which are present at the outermost shell of an atom are called valence electrons and valency of an electron is the number of electrons in which atom accepts or donate to form a bond.
We know that the valence electrons of phosphorus is five and there are five fluoride bonds to the central metal atom.
The steric number of PF5 can be calculated as,
Stericnumber=2valenceelectronofcentralatom+no.of.bondedatom
In PF5 molecule, valence electron of central atom is 5 and phosphorus is surrounded by 5 fluorine atoms so the no. of bonded atom in PF5 is 5. Substituting the values in formula we get,
Stericnumber=25+5=5
The steric number of PF5 is five.
The structure of PF5 is,
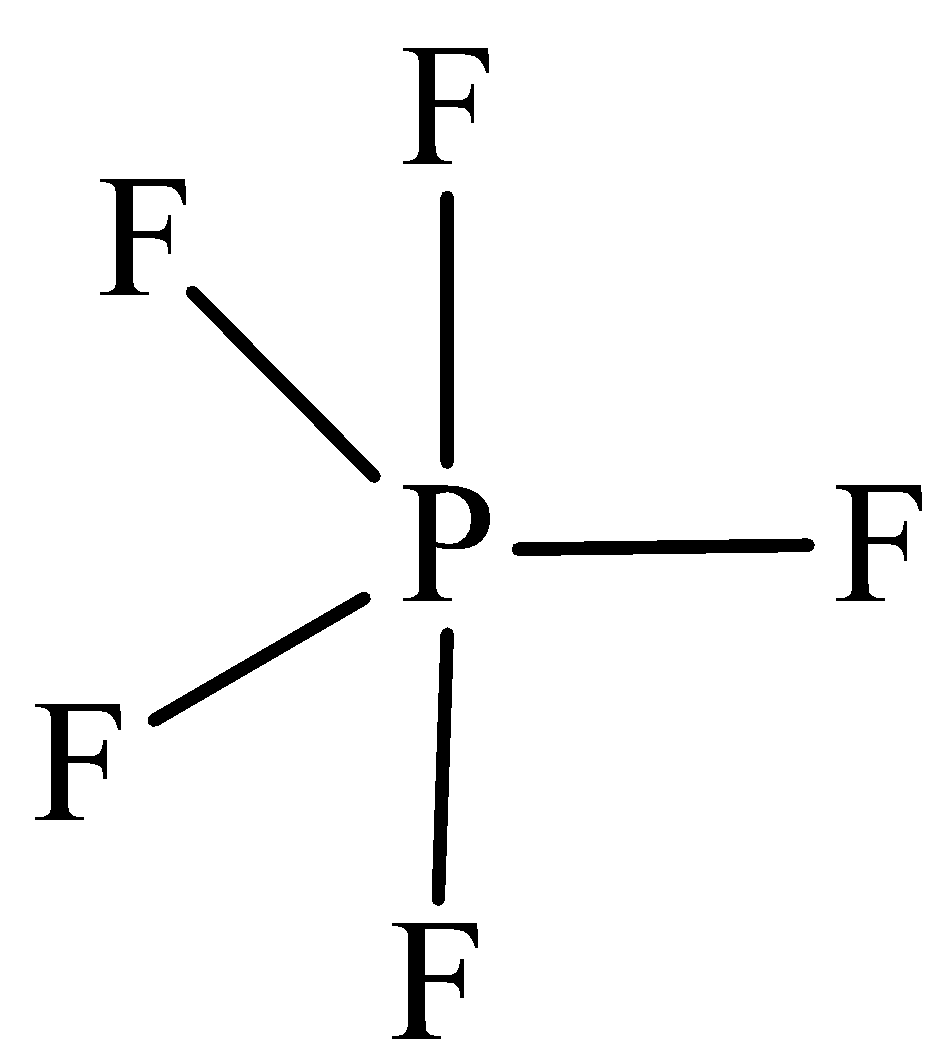
In PF5 the central atom is phosphorus has five bonding domains. The hybridization of PCl5 is sp3d. The molecular geometry is trigonal bipyramidal.
The sp3d hybridization:
The combination of 1s,3p and 1d orbital result in the formation of sp3d orbital in which three lobes are oriented towards the corners of a triangle and the other lie perpendicular to them to minimize the repulsions.
So, the correct answer is Option C .
Note:
We can also calculate the steric number as follow,
The steric number is a sum of the number of ligands and lone pairs surrounding the central atom.
stericnumber = (m + n)
Where m is the number of ligands and n is the number of lone pairs.
