Question
Question: The structure of major product X and Y formed in the following reaction are: $CHCl_3 \xrightarrow[Li...
The structure of major product X and Y formed in the following reaction are: CHCl3O2Light(X)C6H6(excess)AlCl3(Y)
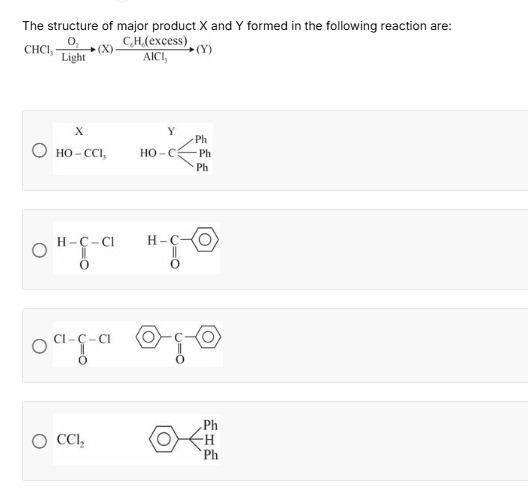
HO-CCl3
Phosgene
Phosgene
CCl2
The third option matches the derived products where X is phosgene (COCl2) and Y is benzophenone (Ph−CO−Ph).
Solution
Step-by-step Derivations:
1. Formation of Product X: The first reaction involves the oxidation of chloroform (CHCl3) in the presence of oxygen (O2) and light. This is a well-known reaction where chloroform decomposes to form phosgene (COCl2). This reaction is a significant safety concern as phosgene is a highly toxic gas. The reaction can be written as: 2CHCl3+O2Light2COCl2+2HCl Therefore, the major product X is phosgene (COCl2).
2. Formation of Product Y: The second reaction involves the reaction of product X (COCl2) with excess benzene (C6H6) in the presence of aluminum chloride (AlCl3). This is a Friedel-Crafts acylation reaction. Phosgene (COCl2) is a di-acyl chloride, meaning it has two acyl chloride functionalities.
-
First Acylation: Phosgene reacts with one molecule of benzene to form benzoyl chloride. Cl−C(=O)−Cl+C6H6AlCl3C6H5−C(=O)−Cl+HCl (Benzoyl chloride)
-
Second Acylation: Since benzene is in excess, the benzoyl chloride formed can further undergo Friedel-Crafts acylation with another molecule of benzene. C6H5−C(=O)−Cl+C6H6AlCl3C6H5−C(=O)−C6H5+HCl (Benzophenone)
Therefore, the major product Y is benzophenone (C6H5−C(=O)−C6H5 or Ph−CO−Ph).
Conclusion: Based on the analysis, product X is COCl2 and product Y is Ph−CO−Ph.
