Question
Question: The structure of intermediate A in the following reaction is : 
a.) 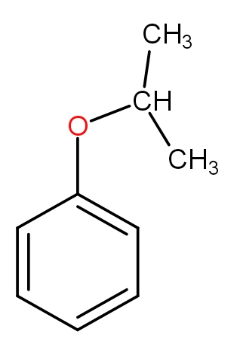
b.) 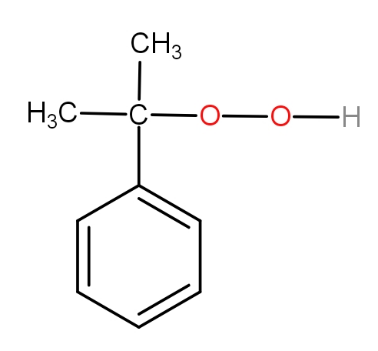
c.) 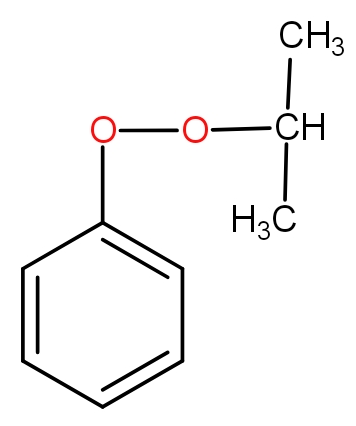
d.) 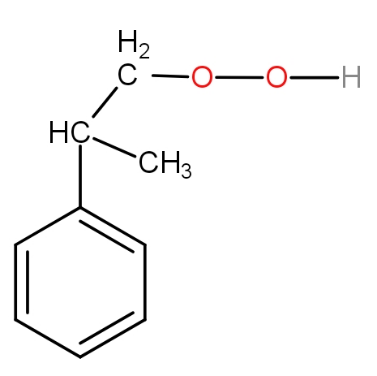
Solution
. The reaction above is of cumene in which the dioxygen molecule gets incorporated in the molecule by forming bonds with tertiary carbon present. So, the intermediate involves an intermediate which has a carbon-oxygen bond at tertiary carbon. The intermediate has molecular mass 152.19 g/mol.
Complete step by step answer:
The substrate given to us is cumene. The first step involves reagent oxygen molecule which normally gets incorporated in the molecule and thus do oxidation of the molecule. Cumene reacts with oxygen molecules and leads to formation of cumene hydroperoxide which is A. This cumene hydroperoxide on hydrolysis gives phenol and acetone. The reaction is as -
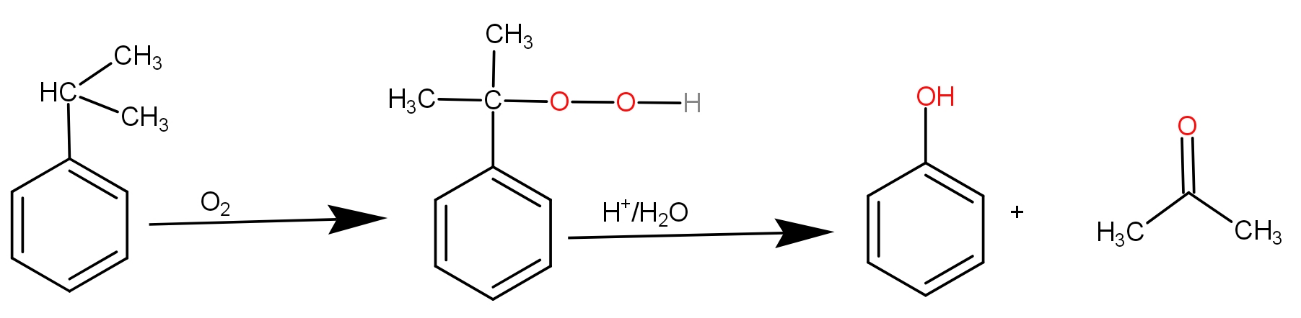
So, the correct answer is “Option B”.
Additional Information:
Cumene has another name isopropyl benzene is a flammable colourless liquid. It is a constituent of crude oil and refined fuel. Most of the cumene is converted to cumene hydroperoxide and this cumene hydroperoxide acts as catalyst for the oxidation of many compounds.
The synthesis of cumene is by Friedel Crafts alkylation reaction of benzene in which the benzene gets substituted by propylene. This cumene if given long exposure to air gives cumene peroxides.
Note: Cumene hydroperoxide is used as an oxidizing agent. It is even involved as an organic peroxide for manufacturing of propylene oxide by oxidation of propylene. It is colourless liquid with sharp and irritating odour. It is used in production of acetone and phenol as a polymerization catalyst.
