Question
Question: The structure of diborane contains. A. four \[2c - 2e\] bonds and two \[3c - 2e\] bonds B two \[...
The structure of diborane contains.
A. four 2c−2e bonds and two 3c−2e bonds
B two 2c−2e bonds and four 3c−2e bonds
C. two 2c−2e bonds and two 3c−2e bonds
D. four 2c−2e bonds and four 3c−2e bonds
Solution
Boron is sp3 hybridized, so the total number of bonds formed by each boron atom is four. Also consider the total number of electrons involved in bonding will determine the type of bonding.
Complete step by step answer:
Boron is an element in the periodic table with atomic number 5. The electronic configuration of boron is:
B: 1s22s22p1 (in ground state)
B: 1s22s12px12py1 (in excited state).
The valence shell configuration of boron orbitals can be shown as:
B in ground state: 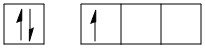
B in hybridized state: 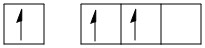
The bonding of the boron in B2H6 can be shown as:
B2H6: 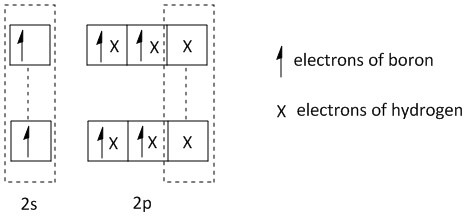
Clearly from the above structure of orbitals, two different types of bonding orbitals are present in B2H6 molecules. The hybridization of the boron atom is sp3. Four of the hybrid orbitals are forming sigma bonds directly to the hydrogen atoms. The electrons involved in this type of bond are one electron from boron and one electron from hydrogen. So it is a two centre two electron bond.
The other two hybrid orbitals form two bridging bonds between the central boron atoms. This type of bridging bond is also known as a banana bond consisting of B−H−B bonds. Here one electron each from two boron atoms and one electron from the hydrogen atom are involved. So it is a three centre two electron bond.
Thus the correct option is A, i.e. four 2c−2e bonds and two 3c−2e bonds.
Note:
In general the electron deficient bonds are bridge bonds. The number of electrons involved in such bonds is less than the normal. Boron and aluminium are known to form such compounds by sharing electrons from bonded atoms.
