Question
Question: The structure of di-chloromethane is a.Tetrahedral b.Trigonal c.Linear d.Hexagonal...
The structure of di-chloromethane is
a.Tetrahedral
b.Trigonal
c.Linear
d.Hexagonal
Solution
We know that dichloromethane is an organochlorine compound with the formula CH2Cl2. It is a colourless, volatile liquid with chloroform- like sweet odour is widely used as a solvent. We know that it is non-combustible but if exposed to high temperatures it may produce toxic chloride fumes.
Complete step by step answer:
We should know that the other name of di-chloromethane is Methylene chloride or Methylene dichloride.
The structure of dichloromethane is tetrahedral as it is sp3 hybridized.
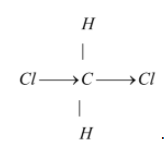
So it has a tetrahedral structure.
Hence the correct option is (a) tetrahedral.
Note:
We should know that even though CH2Cl12 is not miscible in water, it can be dissolved in a wide range of organic solvents. This property of dichloromethane is combined with its volatility, making DCM a highly effective solvent in most industrial processes. It is also commonly used as a paint remover.
